Preparation of Fe3O4@SW-MIL-101-NH2 for selective pre-concentration of chlorogenic acid metabolites in rat plasma, urine, and feces samples
- PMID: 36105170
- PMCID: PMC9463528
- DOI: 10.1016/j.jpha.2022.01.002
Preparation of Fe3O4@SW-MIL-101-NH2 for selective pre-concentration of chlorogenic acid metabolites in rat plasma, urine, and feces samples
Abstract
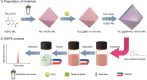
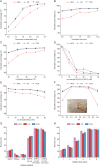
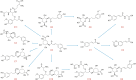
An innovative sandwich-structural Fe-based metal-organic framework magnetic material (Fe3O4@SW-MIL-101-NH2) was fabricated using a facile solvothermal method. The characteristic properties of the material were investigated by field emission scanning electron microscopy, transmission electron microscopy (TEM), energy-dispersive X-ray spectroscopy, Fourier transform infrared spectroscopy, X-ray powder diffraction, vibrating sample magnetometry, and Brunauer-Emmett-Teller measurements. Fe3O4@SW-MIL-101-NH2 is associated with advantages, such as robust magnetic properties, high specific surface area, and satisfactory storage stability, as well as good selective recognition ability for chlorogenic acid (CA) and its metabolites via chelation, hydrogen bonding, and π-interaction. The results of the static adsorption experiment indicated that Fe3O4@SW-MIL-101-NH2 possessed a high adsorption capacity toward CA and its isomers, cryptochlorogenic acid (CCA) and neochlorogenic acid (NCA), and the adsorption behaviors were fitted using the Langmuir adsorption isotherm model. Then, a strategy using magnetic solid-phase extraction (MSPE) and ultra-performance liquid chromatography coupled with quadrupole time-of-flight tandem mass spectrometry (UPLC-Q-TOF MS/MS) was developed and successfully employed for the selective pre-concentration and rapid identification of CA metabolites in rat plasma, urine, and feces samples. This work presents a prospective strategy for the synthesis of magnetic adsorbents and the high-efficiency pretreatment of CA metabolites.
Keywords: Chlorogenic acid; Magnetic solid-phase extraction; Metabolic pathway; Metal-organic framework; Sandwich structure.
© 2022 The Authors.
Figures


Similar articles
-
Preparation of magnetic yolk-shell structured metal-organic framework material and its application in pharmacokinetics study of alkaloids.Anal Bioanal Chem. 2021 Nov;413(28):6987-6999. doi: 10.1007/s00216-021-03656-2. Epub 2021 Sep 17. Anal Bioanal Chem. 2021. PMID: 34535814
-
Molecularly imprinted polymers based on magnetic metal-organic frameworks for surface-assisted laser desorption/ionization time-of-flight mass spectrometry analysis and simultaneous luteolin enrichment.J Chromatogr A. 2022 Aug 16;1678:463377. doi: 10.1016/j.chroma.2022.463377. Epub 2022 Jul 26. J Chromatogr A. 2022. PMID: 35926390
-
[Determination of three diphenyl ether herbicides in rice by magnetic solid phase extraction using Fe3O4@MOF-808 coupled with high performance liquid chromatography].Se Pu. 2021 Mar;39(3):316-323. doi: 10.3724/SP.J.1123.2020.06007. Se Pu. 2021. PMID: 34227312 Free PMC article. Chinese.
-
Preparation of yolk-shell structure NH2-MIL-125 magnetic nanoparticles for the selective extraction of nucleotides.Mikrochim Acta. 2021 Nov 15;188(12):419. doi: 10.1007/s00604-021-05071-x. Mikrochim Acta. 2021. PMID: 34782919
-
Magnetic materials as sorbents for metal/metalloid preconcentration and/or separation. A review.Anal Chim Acta. 2013 Jul 30;789:1-16. doi: 10.1016/j.aca.2013.04.021. Epub 2013 Apr 29. Anal Chim Acta. 2013. PMID: 23856225 Review.
Cited by
-
Ultra-High Adsorption Capacity of Core-Shell-Derived Magnetic Zeolite Imidazolate Framework-67 as Adsorbent for Selective Extraction of Theophylline.Molecules. 2023 Jul 21;28(14):5573. doi: 10.3390/molecules28145573. Molecules. 2023. PMID: 37513444 Free PMC article.
References
-
- Nakatani N., Kayano S., Kikuzaki H., et al. Identification, quantitative determination, and antioxidative activities of chlorogenic acid isomers in prune (Prunus domestica L.) J. Agric. Food Chem. 2000;48:5512–5516. - PubMed
-
- Li N., Gao X.-Y., Fan Q., et al. Rapid determination of chlorogenic acid in aqueous solution of Flos Lonicerae Japonicae extraction. Zhongguo Zhongyao Zazhi. 2007;32:312–314. - PubMed
-
- Arai K., Terashima H., Aizawa S., et al. Simultaneous determination of trigonelline, caffeine, chlorogenic acid and their related compounds in instant coffee samples by HPLC using an acidic mobile phase containing octanesulfonate. Anal. Sci. 2015;31:831–835. - PubMed
-
- Sato Y., Itagaki S., Kurokawa T., et al. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int. J. Pharm. 2011;403:136–138. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

