CGX, a standardized herbal syrup, inhibits colon-liver metastasis by regulating the hepatic microenvironments in a splenic injection mouse model
- PMID: 36105183
- PMCID: PMC9465806
- DOI: 10.3389/fphar.2022.906752
CGX, a standardized herbal syrup, inhibits colon-liver metastasis by regulating the hepatic microenvironments in a splenic injection mouse model
Abstract
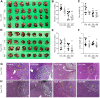
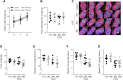
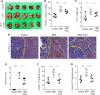
Background: Colon-liver metastasis is observed in approximately 50% of patients with colorectal cancer and is a critical risk factor for a low survival rate. Several clinical studies have reported that colon-liver metastasis is accelerated by pathological hepatic microenvironments such as hepatic steatosis or fibrosis. Chunggan syrup (CGX), a standardized 13-herbal mixture, has been prescribed to patients with chronic liver diseases, including fatty liver, inflammation and fibrotic change, based on preclinical and clinical evidence. Aim of the study: In the present study, we investigated anti-liver metastatic the effects of CGX in a murine colon carcinoma (MC38)-splenic injection mouse model. Materials and methods: C57BL/6N mice were administered with CGX (100, 200 or 400 mg/kg) for 14 days before or after MC38-splenic injection under normal and high-fat diet (HFD) fed conditions. Also, above experiment was repeated without MC38-splenic injection to explore underlying mechanism. Results: The number of tumor nodules and liver weight with tumors were sup-pressed by preadministration of CGX in both normal and HFD fed mice. Regarding its mechanisms, we found that CGX administration significantly activated epithelial-cadherin (E-cadherin), but decreased vascular endothelial-cadherin (VE-cadherin) in hepatic tissues under MC38-free conditions. In addition, CGX administration significantly reduced hepatic steatosis, via modulation of lipolytic and lipogenic molecules, including activated adenosine monophosphate activated protein kinase (AMPK) and peroxisome proliferator activated receptor-alpha (PPARα). Conclusion: The present data indicate that CGX exerts an anti-colon-liver metastatic property via modulation of hepatic lipid related microenvironments.
Keywords: chuggan syrup (CGX); colon-liver metastasis; hepatic microenvironment; hepatic steatosis; herbal medicine.
Copyright © 2022 Lee, Hwang and Son.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
The herbal formula CGX ameliorates the expression of vascular endothelial growth factor in alcoholic liver fibrosis.J Ethnopharmacol. 2013 Dec 12;150(3):892-900. doi: 10.1016/j.jep.2013.09.035. Epub 2013 Oct 1. J Ethnopharmacol. 2013. PMID: 24095833
-
Chunggan extract (CGX), methionine-and choline-deficient (MCD) diet-induced hepatosteatosis and oxidative stress in C57BL/6 mice.Hum Exp Toxicol. 2013 Dec;32(12):1258-69. doi: 10.1177/0960327113485253. Epub 2013 Aug 22. Hum Exp Toxicol. 2013. PMID: 23970447
-
Antifibrotic effects of CGX, a traditional herbal formula, and its mechanisms in rats.J Ethnopharmacol. 2010 Feb 3;127(2):534-42. doi: 10.1016/j.jep.2009.10.001. Epub 2009 Oct 13. J Ethnopharmacol. 2010. PMID: 19833189
-
The ménage à trois of autophagy, lipid droplets and liver disease.Autophagy. 2022 Jan;18(1):50-72. doi: 10.1080/15548627.2021.1895658. Epub 2021 Apr 2. Autophagy. 2022. PMID: 33794741 Free PMC article. Review.
-
A Review of Herbal Resources Inducing Anti-Liver Metastasis Effects in Gastrointestinal Tumors via Modulation of Tumor Microenvironments in Animal Models.Cancers (Basel). 2023 Jun 29;15(13):3415. doi: 10.3390/cancers15133415. Cancers (Basel). 2023. PMID: 37444525 Free PMC article. Review.
References
-
- Baghban R., Roshangar L., Jahanban-Esfahlan R., Seidi K., Ebrahimi-Kalan A., Jaymand M., et al. (2020). Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun. Signal. 18, 59. 10.1186/s12964-020-0530-4 PubMed Abstract | 10.1186/s12964-020-0530-4 | Google Scholar - DOI - DOI - PMC - PubMed
-
- Beloribi-Djefaflia S., Vasseur S., Guillaumond F. (2016). Lipid metabolic reprogramming in cancer cells. Oncogenesis 5, e189. 10.1038/oncsis.2015.49 PubMed Abstract | 10.1038/oncsis.2015.49 | Google Scholar - DOI - DOI - PMC - PubMed
-
- Brodt P. (2016). Role of the microenvironment in liver metastasis: From pre-to prometastatic niches. Clin. Cancer Res. 22, 5971–5982. 10.1158/1078-0432.CCR-16-0460 PubMed Abstract | 10.1158/1078-0432.CCR-16-0460 | Google Scholar - DOI - DOI - PubMed
-
- Carr T. P., Andresen C. J., Rudel L. L. (1993). Enzymatic determination of triglyceride, free cholesterol, and total cholesterol in tissue lipid extracts. Clin. Biochem. 26, 39–42. 10.1016/0009-9120(93)90015-x PubMed Abstract | 10.1016/0009-9120(93)90015-x | Google Scholar - DOI - DOI - PubMed
-
- Chandra R., Karalis J. D., Liu C., Murimwa G. Z., Voth Park J., Heid C. A., et al. (2021). The colorectal cancer tumor microenvironment and its impact on liver and lung metastasis. Cancers 13, 6206. 10.3390/cancers13246206 PubMed Abstract | 10.3390/cancers13246206 | Google Scholar - DOI - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

