Effect of Silibinin on Dyslipidemia and Glycemic Alteration Associated with Polycystic Ovarian Syndrome: An Experimental Study on Rats
- PMID: 36105429
- PMCID: PMC9464776
- DOI: 10.2147/DMSO.S377404
Effect of Silibinin on Dyslipidemia and Glycemic Alteration Associated with Polycystic Ovarian Syndrome: An Experimental Study on Rats
Abstract
Purpose: Females with polycystic ovary syndrome (PCOS) are found to have hormonal and metabolic alterations. This study investigated the efficacy of the flavonolignan silibinin in restoring the metabolic alterations associated with letrozole-induced PCOS in rats.
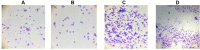
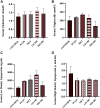
Methods: The study allocated 42 albino rats into two groups. The first group was a normal control group (n=12) in which only the vehicle was given. The second group, the PCOS group (n=30), received letrozole (1 mg/kg/day) orally for 21 days. On day 21, six animals from the first group and six animals from the second group were sacrificed to confirm the development of PCOS, and the rest of the animals (n=24) in the second group were distributed equally into four groups: the PCOS group received vehicle, the metformin (MET) group received 300 mg/kg metformin orally, and the low-dose silibinin (LD-100) and high-dose silibinin (HD-200) groups received 100 and 200 mg/kg silibinin intraperitoneally, respectively. Blockade of the estrus cycle in the diestrus phase, hyperglycemia, and body weight elevation were related to a positive PCOS induction. An oral glucose tolerance test (OGTT) was also carried out for all animals on day 21 and on the last day of the experiment (day 40) to investigate the effect of silibinin on insulin resistance. The rats' lipid profile, insulin level, estrus cycle patterns, body weight, and weights of the ovaries and uterus were also measured on day 40.
Results: A 19-day silibinin treatment led to the restoration of regular estrus cyclicity, reduced the glucose spike in OGTT analysis, and alleviated insulin resistance in PCOS rats. There was a statistically non-significant decrement in insulin level and lipid profile in the treatment groups.
Conclusion: Silibinin flavonolignan ameliorated some metabolic and reproductive alterations associated with PCOS. This could be related to the decreased insulin resistance, and antiandrogenic and phytoestrogenic activity of silibinin. Further study with longer term therapy is recommended to clarify more potential effects of silibinin and its mechanism of action in PCOS.
Keywords: impaired glucose tolerance; lipid profile; metabolic alteration; silibinin.
© 2022 Marouf.
Conflict of interest statement
The author reports no conflicts of interest in this work.
Figures



Similar articles
-
Therapeutic Effects of Silibinin Against Polycystic Ovary Syndrome Induced by Letrozole in Rats via Its Potential Anti-Inflammatory and Anti-Oxidant Activities.J Inflamm Res. 2022 Sep 9;15:5185-5199. doi: 10.2147/JIR.S379725. eCollection 2022. J Inflamm Res. 2022. PMID: 36110507 Free PMC article.
-
Partially purified non-polar phytocomponents from Aloe barbadensis Mill. gel restores metabolic and reproductive comorbidities in letrozole-induced polycystic ovary syndrome rodent model- an "in-vivo" study.J Ethnopharmacol. 2022 Jun 12;291:115161. doi: 10.1016/j.jep.2022.115161. Epub 2022 Mar 7. J Ethnopharmacol. 2022. PMID: 35271948
-
Therapeutic exploration of polyherbal formulation against letrozole induced PCOS rats: A mechanistic approach.Heliyon. 2023 Apr 21;9(5):e15488. doi: 10.1016/j.heliyon.2023.e15488. eCollection 2023 May. Heliyon. 2023. PMID: 37180914 Free PMC article.
-
Pathophysiological changes in experimental polycystic ovary syndrome in female albino rats: Using either hemin or L-arginine.J Cell Physiol. 2019 Jun;234(6):8426-8435. doi: 10.1002/jcp.27757. Epub 2018 Nov 15. J Cell Physiol. 2019. PMID: 30443939 Review.
-
AMERICAN ASSOCIATION OF CLINICAL ENDOCRINOLOGISTS, AMERICAN COLLEGE OF ENDOCRINOLOGY, AND ANDROGEN EXCESS AND PCOS SOCIETY DISEASE STATE CLINICAL REVIEW: GUIDE TO THE BEST PRACTICES IN THE EVALUATION AND TREATMENT OF POLYCYSTIC OVARY SYNDROME - PART 2.Endocr Pract. 2015 Dec;21(12):1415-26. doi: 10.4158/EP15748.DSCPT2. Endocr Pract. 2015. PMID: 26642102 Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

