Quercetin Protects against MPP+/MPTP-Induced Dopaminergic Neuron Death in Parkinson's Disease by Inhibiting Ferroptosis
- PMID: 36105483
- PMCID: PMC9467739
- DOI: 10.1155/2022/7769355
Quercetin Protects against MPP+/MPTP-Induced Dopaminergic Neuron Death in Parkinson's Disease by Inhibiting Ferroptosis
Abstract
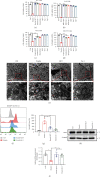
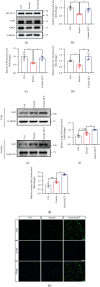
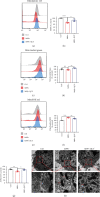
Ferroptosis, a novel form of regulated cell death, is caused by accumulation of lipid peroxides and excessive iron deposition. This process has been linked to the death of dopaminergic neurons in substantia nigra compacta (SNc) of Parkinson's disease (PD) patients. Quercetin (QCT), a natural flavonoid, has multiple pharmacological activities. However, it has not been established whether QCT can protect against dopaminergic neuron death by inhibiting ferroptosis. In this study, we investigated the potential antiferroptotic effects of QCT in cellular models established using specific ferroptosis inducers (Erastin and RSL-3) and MPP+. The effects were also explored using MPTP-induced PD mouse models. The cell counting kit-8 (CCK-8) assay was performed to assess cell viability. Variations in mitochondrial morphology were evaluated by transmission electron microscopy (TEM) while the mitochondrial membrane potential, mass, and ROS were measured by fluorescent probes. Lipid peroxidation levels were assayed through measurement of lipid ROS, MDA, GSH, and SOD levels. The effects of QCT on MPTP-induced behavioral disorders were examined by rotarod and open field tests. In vitro and in vivo, QCT significantly inhibited ferroptosis by activating the nuclear factor erythroid 2-related factor 2 (Nrf2) protein. Additionally, QCT ameliorated motor behavioral impairments and protected against the loss of dopaminergic neurons in MPTP-induced PD models. Interestingly, Nrf2 knockdown alleviated the protective effects of QCT against ferroptosis. In conclusion, these results demonstrate that ferroptosis is involved in MPP+/MPTP-induced PD, and QCT inhibits ferroptosis by activating the Nrf2 protein. Therefore, QCT is a potential agent for preventing the loss of dopaminergic neurons by targeting ferroptosis.
Copyright © 2022 Zhi-Hao Lin et al.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

