Integration of genomic variants and bioinformatic-based approach to drive drug repurposing for multiple sclerosis
- PMID: 36105612
- PMCID: PMC9464879
- DOI: 10.1016/j.bbrep.2022.101337
Integration of genomic variants and bioinformatic-based approach to drive drug repurposing for multiple sclerosis
Abstract
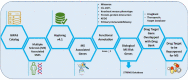
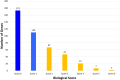
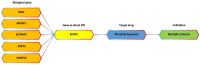
Multiple sclerosis (MS) is a chronic autoimmune disease in the central nervous system (CNS) marked by inflammation, demyelination, and axonal loss. Currently available MS medication is limited, thereby calling for a strategy to accelerate new drug discovery. One of the strategies to discover new drugs is to utilize old drugs for new indications, an approach known as drug repurposing. Herein, we first identified 421 MS-associated SNPs from the Genome-Wide Association Study (GWAS) catalog (p-value < 5 × 10-8), and a total of 427 risk genes associated with MS using HaploReg version 4.1 under the criterion r2 > 0.8. MS risk genes were then prioritized using bioinformatics analysis to identify biological MS risk genes. The prioritization was performed based on six defined categories of functional annotations, namely missense mutation, cis-expression quantitative trait locus (cis-eQTL), molecular pathway analysis, protein-protein interaction (PPI), genes overlap with knockout mouse phenotype, and primary immunodeficiency (PID). A total of 144 biological MS risk genes were found and mapped into 194 genes within an expanded PPI network. According to the DrugBank and the Therapeutic Target Database, 27 genes within the list targeted by 68 new candidate drugs were identified. Importantly, the power of our approach is confirmed with the identification of a known approved drug (dimethyl fumarate) for MS. Based on additional data from ClinicalTrials.gov, eight drugs targeting eight distinct genes are prioritized with clinical evidence for MS disease treatment. Notably, CD80 and CD86 pathways are promising targets for MS drug repurposing. Using in silico drug repurposing, we identified belatacept as a promising MS drug candidate. Overall, this study emphasized the integration of functional genomic variants and bioinformatic-based approach that reveal important biological insights for MS and drive drug repurposing efforts for the treatment of this devastating disease.
Keywords: Autoimmune disease; Bioinformatics; Drug repurposing; Genomic variants; Multiple sclerosis.
© 2022 The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Integration of genetic variants and gene network for drug repurposing in colorectal cancer.Pharmacol Res. 2020 Nov;161:105203. doi: 10.1016/j.phrs.2020.105203. Epub 2020 Sep 17. Pharmacol Res. 2020. PMID: 32950641
-
Drug Repurposing for Atopic Dermatitis by Integration of Gene Networking and Genomic Information.Front Immunol. 2021 Oct 13;12:724277. doi: 10.3389/fimmu.2021.724277. eCollection 2021. Front Immunol. 2021. PMID: 34721386 Free PMC article.
-
Genomic variants-driven drug repurposing for tuberculosis by utilizing the established bioinformatic-based approach.Biochem Biophys Rep. 2022 Aug 31;32:101334. doi: 10.1016/j.bbrep.2022.101334. eCollection 2022 Dec. Biochem Biophys Rep. 2022. PMID: 36090591 Free PMC article.
-
The use of genomic variants to drive drug repurposing for chronic hepatitis B.Biochem Biophys Rep. 2022 Jul 8;31:101307. doi: 10.1016/j.bbrep.2022.101307. eCollection 2022 Sep. Biochem Biophys Rep. 2022. PMID: 35832745 Free PMC article.
-
Reworking GWAS Data to Understand the Role of Nongenetic Factors in MS Etiopathogenesis.Genes (Basel). 2020 Jan 14;11(1):97. doi: 10.3390/genes11010097. Genes (Basel). 2020. PMID: 31947683 Free PMC article. Review.
Cited by
-
Genetic susceptibility and causal pathway analysis of eye disorders coexisting in multiple sclerosis.Front Immunol. 2024 Feb 5;15:1337528. doi: 10.3389/fimmu.2024.1337528. eCollection 2024. Front Immunol. 2024. PMID: 38375484 Free PMC article.
-
Neurodegenerative Diseases: Can Caffeine Be a Powerful Ally to Weaken Neuroinflammation?Int J Mol Sci. 2022 Oct 26;23(21):12958. doi: 10.3390/ijms232112958. Int J Mol Sci. 2022. PMID: 36361750 Free PMC article. Review.
-
Leveraging Genomic and Bioinformatic Analysis to Enhance Drug Repositioning for Dermatomyositis.Bioengineering (Basel). 2023 Jul 27;10(8):890. doi: 10.3390/bioengineering10080890. Bioengineering (Basel). 2023. PMID: 37627776 Free PMC article.
-
A genomic and bioinformatic-based approach to identify genetic variants for liver cancer across multiple continents.Genomics Inform. 2023 Dec;21(4):e48. doi: 10.5808/gi.23067. Epub 2023 Dec 29. Genomics Inform. 2023. PMID: 38224715 Free PMC article.
-
The landscape of the methodology in drug repurposing using human genomic data: a systematic review.Brief Bioinform. 2024 Jan 22;25(2):bbad527. doi: 10.1093/bib/bbad527. Brief Bioinform. 2024. PMID: 38279645 Free PMC article.
References
LinkOut - more resources
Full Text Sources

