A Streptomyces P450 enzyme dimerizes isoflavones from plants
- PMID: 36105730
- PMCID: PMC9443421
- DOI: 10.3762/bjoc.18.113
A Streptomyces P450 enzyme dimerizes isoflavones from plants
Abstract
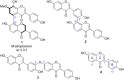
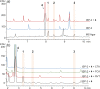
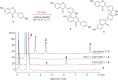
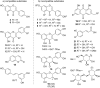
Dimerization is a widespread natural strategy that enables rapid structural diversification of natural products. However, our understanding of the dimerization enzymes involved in this biotransformation is still limited compared to the numerous reported dimeric natural products. Here, we report the characterization of three new isoflavone dimers from Streptomyces cattleya cultured on an isoflavone-containing agar plate. We further identified a cytochrome P450 monooxygenase, CYP158C1, which is able to catalyze the dimerization of isoflavones. CYP158C1 can also dimerize plant-derived polyketides, such as flavonoids and stilbenes. Our work represents a unique bacterial P450 that can dimerize plant polyphenols, which extends the insights into P450-mediated biaryl coupling reactions in biosynthesis.
Keywords: biaryl coupling; cytochrome P450; dimerization; isoflavone; natural product.
Copyright © 2022, Liu et al.
Figures
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
