Efficacy and safety of concomitant immunotherapy and denosumab in patients with advanced non-small cell lung cancer carrying bone metastases: A retrospective chart review
- PMID: 36105807
- PMCID: PMC9464943
- DOI: 10.3389/fimmu.2022.908436
Efficacy and safety of concomitant immunotherapy and denosumab in patients with advanced non-small cell lung cancer carrying bone metastases: A retrospective chart review
Abstract
Background: Synergistic anti-tumor effects were observed in vivo and in vitro when immune checkpoint inhibitors (ICIs) were combined with denosumab. However, the clinical benefit and safety of this synergy have not been adequately evaluated in non-small cell lung cancer (NSCLC).
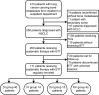
Methods: Consecutive charts of NSCLC patients with bone metastases between December 2020 and December 2021 in the Chinese National Cancer Center were reviewed. The entire cohort was divided into one experimental group (denosumab + ICIs [DI]) and three control groups (denosumab + non-ICIs [DnI], phosphates + ICIs [PI], phosphates + non-ICIs [PnI]). Real-world objective response rates (ORRs), median progression-free survival (mPFS), skeletal-related events (SREs), and adverse events (AEs) were compared between groups.
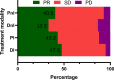
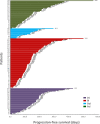
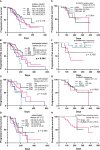
Results: A total of 171/410 (41.7%) patients with advanced or recurrent NSCLC carrying bone metastases who received bone-targeted therapy were eligible for analysis. Although the DI group showed a better benefit trend, differences were not statistically significant concerning the therapeutic efficacy among the DI group (n = 40), PI group (n = 74), DnI group (n = 15), and PnI group (n = 42) (ORRs: 47.5%, 43.2%, 33.3%, and 40.5%, respectively, p = 0.799; and mPFS: 378, 190, 170, and 172 days, respectively, p = 0.115; SREs: 5%, 10.8%, 13.3%, and 11.9%, respectively, p = 0.733). Nevertheless, further analysis in the NON-DRIVER cohort revealed a greater benefit for the DI group (p = 0.045). Additionally, the AEs of the DI group were not significantly different from those of the PI, DnI, and PnI groups (AEs: 27.5%, 39.2%, 26.7%, and 28.6%, respectively, p = 0.742). Furthermore, the multivariate analysis revealed the independent prognostic role of DI treatment for PFS in the overall cohort. Within the DI group, we did not observe differences in benefit among different mutational subgroups (p = 0.814), but patients with single-site bone metastasis (p = 0.319) and high PD-L1 expression (p = 0.100) appeared to benefit more, though no significant differences were observed.
Conclusions: Denosumab exhibited synergistic antitumor efficacy without increasing toxicity when used concomitantly with ICIs in patients with advanced non-small cell lung cancer carrying bone metastases.
Keywords: bone metastases; denosumab; efficacy; immunotherapy; non-small cell lung cancer; safety; synergistic efficacy.
Copyright © 2022 Li, Lei, Li, Xing, Hao, Xu, Xu and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Immune Checkpoint Inhibitors With or Without Bone-Targeted Therapy in NSCLC Patients With Bone Metastases and Prognostic Significance of Neutrophil-to-Lymphocyte Ratio.Front Immunol. 2021 Nov 10;12:697298. doi: 10.3389/fimmu.2021.697298. eCollection 2021. Front Immunol. 2021. PMID: 34858389 Free PMC article.
-
Combination therapy with immune checkpoint inhibitors and denosumab improves clinical outcomes in non-small cell lung cancer with bone metastases.Lung Cancer. 2024 Jul;193:107858. doi: 10.1016/j.lungcan.2024.107858. Epub 2024 Jun 19. Lung Cancer. 2024. PMID: 38901176
-
The Therapeutic Effect and Clinical Outcome of Immune Checkpoint Inhibitors on Bone Metastasis in Advanced Non-Small-Cell Lung Cancer.Front Oncol. 2022 Apr 1;12:871675. doi: 10.3389/fonc.2022.871675. eCollection 2022. Front Oncol. 2022. PMID: 35433422 Free PMC article.
-
Osteonecrosis of the jaw in a metastatic lung cancer patient with bone metastases undergoing pembrolizumab + denosumab combination therapy: Case report and literature review.Oral Oncol. 2020 Dec;111:104874. doi: 10.1016/j.oraloncology.2020.104874. Epub 2020 Jun 27. Oral Oncol. 2020. PMID: 32605876 Review.
-
Efficacy and Safety of Combined Brain Radiotherapy and Immunotherapy in Non-Small-Cell Lung Cancer With Brain Metastases: A Systematic Review and Meta-Analysis.Clin Lung Cancer. 2022 Mar;23(2):95-107. doi: 10.1016/j.cllc.2021.06.009. Epub 2021 Jun 24. Clin Lung Cancer. 2022. PMID: 34284948
Cited by
-
Novel predictors of immune checkpoint inhibitor response and prognosis in advanced non-small-cell lung cancer with bone metastasis.Cancer Med. 2023 Jun;12(11):12425-12437. doi: 10.1002/cam4.5952. Epub 2023 Apr 19. Cancer Med. 2023. PMID: 37076988 Free PMC article.
-
RANKL acts an unfavorable prognostic biomarker and potential target in advanced KRAS-mutated lung adenocarcinoma.Thorac Cancer. 2023 May;14(15):1368-1382. doi: 10.1111/1759-7714.14882. Epub 2023 Apr 6. Thorac Cancer. 2023. PMID: 37021520 Free PMC article.
-
Treatment strategies for rapid recurrence and progression after surgery for stage I pulmonary pleomorphic carcinoma: a case report and literature review.Front Immunol. 2025 Jun 4;16:1547872. doi: 10.3389/fimmu.2025.1547872. eCollection 2025. Front Immunol. 2025. PMID: 40534846 Free PMC article. Review.
-
Establishing patient-derived tumor organoids of bone metastasis from lung adenocarcinoma reveals the transcriptomic changes underlying denosumab treatment.Clin Exp Metastasis. 2024 Dec 30;42(1):8. doi: 10.1007/s10585-024-10321-2. Clin Exp Metastasis. 2024. PMID: 39739069
-
Site-Specific Response and Resistance Patterns in Patients with Advanced Non-Small-Cell Lung Cancer Treated with First-Line Systemic Therapy.Cancers (Basel). 2024 Jun 4;16(11):2136. doi: 10.3390/cancers16112136. Cancers (Basel). 2024. PMID: 38893255 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

