Plasma vesicular miR-155 as a biomarker of immune activation in antiretroviral treated people living with HIV
- PMID: 36105810
- PMCID: PMC9464867
- DOI: 10.3389/fimmu.2022.916599
Plasma vesicular miR-155 as a biomarker of immune activation in antiretroviral treated people living with HIV
Abstract
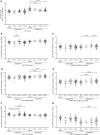
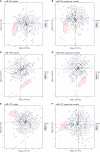
People living with HIV (PLWH), despite suppression of viral replication with antiretroviral therapy (ART), have high morbidity and mortality due to immune activation and chronic inflammation. Discovering new biomarkers of immune activation status under ART will be pertinent to improve PLWH quality of life when the majority will be treated. We stipulate that plasma large and small extracellular vesicle (EVs) and their microRNA content could be easily measured biomarkers to monitor immune activation in PLWH. Venous blood samples from n = 128 ART-treated PLWH with suppressed viral load (≤ 20 copies/mL) and n = 60 HIV-uninfected participants were collected at five testing or treatment centers of PLWH in Burkina Faso. Large and small plasma EVs were purified, counted, and the mature miRNAs miR-29a, miR-146a, and miR-155 were quantified by RT-qPCR. Diagnostic performances of large and small EVs miRNAs level were evaluated by receiver operating characteristic (ROC) curve analysis and principal component analysis (PCA). Among the EVs microRNA measured, only large EVs miR-155 copies distinguished PLWH with immune activation, with AUC of 0.75 for CD4/CD8 < 1 (95% CI: 0.58-0.91, P = 0.0212), and 0.77 for CD8 T cells ≥ 500/µL (95% CI: 0.63-0.92, P = 0.0096). In addition, PCA results suggest that large EVs miR-155 copies may be a biomarker of immune activation. Since miR-155 may influence immune cell function, its enrichment in large EV subpopulations could be a functional biomarker of immune activation in PLWH on ART. This measure could help to monitor and diagnose the immune activation with more accuracy.
Keywords: HIV-1; MicroRNA; biomarker; extracellular vesicles; immune activation; miR-146a; miR-155; miR-29a.
Copyright © 2022 Bazié, Boucher, Goyer, Traoré, Kania, Somé, Alary and Gilbert.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Vesicular MicroRNA as Potential Biomarkers of Viral Rebound.Cells. 2022 Mar 2;11(5):859. doi: 10.3390/cells11050859. Cells. 2022. PMID: 35269481 Free PMC article.
-
Diurnal Variation of Plasma Extracellular Vesicle Is Disrupted in People Living with HIV.Pathogens. 2021 Apr 24;10(5):518. doi: 10.3390/pathogens10050518. Pathogens. 2021. PMID: 33923310 Free PMC article.
-
Plasma Extracellular Vesicle Subtypes May be Useful as Potential Biomarkers of Immune Activation in People With HIV.Pathog Immun. 2021 Jan 14;6(1):1-28. doi: 10.20411/pai.v6i1.384. eCollection 2021. Pathog Immun. 2021. PMID: 33987483 Free PMC article.
-
Assessing inflammation and its role in comorbidities among persons living with HIV.Curr Opin Infect Dis. 2019 Feb;32(1):8-15. doi: 10.1097/QCO.0000000000000510. Curr Opin Infect Dis. 2019. PMID: 30461454 Review.
-
Bidirectional Associations among Nicotine and Tobacco Smoke, NeuroHIV, and Antiretroviral Therapy.J Neuroimmune Pharmacol. 2020 Dec;15(4):694-714. doi: 10.1007/s11481-019-09897-4. Epub 2019 Dec 13. J Neuroimmune Pharmacol. 2020. PMID: 31834620 Free PMC article. Review.
Cited by
-
HIV Replication Increases the Mitochondrial DNA Content of Plasma Extracellular Vesicles.Int J Mol Sci. 2023 Jan 18;24(3):1924. doi: 10.3390/ijms24031924. Int J Mol Sci. 2023. PMID: 36768245 Free PMC article.
-
Development trends of immune activation during HIV infection in recent three decades: a bibliometric analysis based on CiteSpace.Arch Microbiol. 2023 Jul 11;205(8):283. doi: 10.1007/s00203-023-03624-7. Arch Microbiol. 2023. PMID: 37432538
-
HIV-1 diagnostic, prognostic and ART-resistance detection potential of selected MiRNAs.BMC Infect Dis. 2025 Jun 2;25(1):788. doi: 10.1186/s12879-025-11135-7. BMC Infect Dis. 2025. PMID: 40457240 Free PMC article.
-
Immune Cells Release MicroRNA-155 Enriched Extracellular Vesicles That Promote HIV-1 Infection.Cells. 2023 Jan 31;12(3):466. doi: 10.3390/cells12030466. Cells. 2023. PMID: 36766808 Free PMC article.
-
Expression profile of microRNAs related with viral infectivity, inflammatory response, and immune activation in people living with HIV.Front Microbiol. 2023 Mar 2;14:1136718. doi: 10.3389/fmicb.2023.1136718. eCollection 2023. Front Microbiol. 2023. PMID: 36937285 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

