Immune responses following 3rd and 4th doses of heterologous and homologous COVID-19 vaccines in kidney transplant recipients
- PMID: 36105874
- PMCID: PMC9462844
- DOI: 10.1016/j.eclinm.2022.101642
Immune responses following 3rd and 4th doses of heterologous and homologous COVID-19 vaccines in kidney transplant recipients
Abstract
Background: Solid organ transplant recipients have attenuated immune responses to SARS-CoV-2 vaccines. In this study, we report on immune responses to 3rd- (V3) and 4th- (V4) doses of heterologous and homologous vaccines in a kidney transplant population.
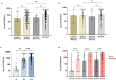
Methods: We undertook a single centre cohort study of 724 kidney transplant recipients prospectively screened for serological responses following 3 primary doses of a SARS-CoV2 vaccine. 322 patients were sampled post-V4 for anti-spike (anti-S), with 69 undergoing assessment of SARS-CoV-2 T-cell responses. All vaccine doses were received post-transplant, only mRNA vaccines were used for V3 and V4 dosing. All participants had serological testing performed post-V2 and at least once prior to their first dose of vaccine.
Findings: 586/724 (80.9%) patients were infection-naïve post-V3; 141/2586 (24.1%) remained seronegative at 31 (21-51) days post-V3. Timing of vaccination in relation to transplantation, OR: 0.28 (0.15-0.54), p=0.0001; immunosuppression burden, OR: 0.22 (0.13-0.37), p<0.0001, and a diagnosis of diabetes, OR: 0.49 (0.32-0.75), p=0.001, remained independent risk factors for non-seroconversion. Seropositive patients post-V3 had greater anti-S if primed with BNT162b2 compared with ChAdOx1, p=0.001.Post-V4, 45/239 (18.8%) infection-naïve patients remained seronegative. De novo seroconversion post-V4 occurred in 15/60 (25.0%) patients. There was no difference in anti-S post-V4 by vaccine combination, p=0.50. T-cell responses were poor, with only 11/54 (20.4%) infection-naive patients having detectable T-cell responses post-V4, with no difference seen by vaccine type.
Interpretation: A significant proportion of transplant recipients remain seronegative following 3- and 4- doses of SARS-CoV-2 vaccines, with poor T-cell responses, and are likely to have inadequate protection against infection. As such alternative strategies are required to provide protection to this vulnerable group.
Funding: MW/PK received study support from Oxford Immunotec.
Keywords: COVID-19; Immunosuppression; Kidney transplant; Vaccination.
© 2022 The Authors.
Conflict of interest statement
DT has received consulting fees and honoraria from AZ and Novartis, SPM has received consulting fees and honoraria from GSK, Vifor, and Celltrion, LL has received consulting fees and honoraria from GSK, BMS, Aurinia, Pfizer, Roche, and Alexion; PK and MW received study support from Oxford Immunotec. Other authors have nothing to disclose.
Figures

References
-
- Willicombe M, Scanlon M, Loud F, Lightstone L. Should we be clinically assessing antibody responses to Covid vaccines in immunocompromised people? BMJ. 2022;377:o966. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

