Micellization: A new principle in the formation of biomolecular condensates
- PMID: 36106018
- PMCID: PMC9465675
- DOI: 10.3389/fmolb.2022.974772
Micellization: A new principle in the formation of biomolecular condensates
Abstract
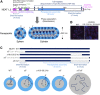
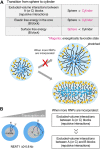
Phase separation is a fundamental mechanism for compartmentalization in cells and leads to the formation of biomolecular condensates, generally containing various RNA molecules. RNAs are biomolecules that can serve as suitable scaffolds for biomolecular condensates and determine their forms and functions. Many studies have focused on biomolecular condensates formed by liquid-liquid phase separation (LLPS), one type of intracellular phase separation mechanism. We recently identified that paraspeckle nuclear bodies use an intracellular phase separation mechanism called micellization of block copolymers in their formation. The paraspeckles are scaffolded by NEAT1_2 long non-coding RNAs (lncRNAs) and their partner RNA-binding proteins (NEAT1_2 RNA-protein complexes [RNPs]). The NEAT1_2 RNPs act as block copolymers and the paraspeckles assemble through micellization. In LLPS, condensates grow without bound as long as components are available and typically have spherical shapes to minimize surface tension. In contrast, the size, shape, and internal morphology of the condensates are more strictly controlled in micellization. Here, we discuss the potential importance and future perspectives of micellization of block copolymers of RNPs in cells, including the construction of designer condensates with optimal internal organization, shape, and size according to design guidelines of block copolymers.
Keywords: NEAT1; architectural RNA (arcRNA); biomolecular condensate; block copolymer (BCP); long non-coding RNA (lncRNA); micellization; paraspeckle; phase separation.
Copyright © 2022 Yamazaki, Yamamoto and Hirose.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Statistical Thermodynamics Approach for Intracellular Phase Separation.Methods Mol Biol. 2022;2509:361-393. doi: 10.1007/978-1-0716-2380-0_22. Methods Mol Biol. 2022. PMID: 35796975
-
Paraspeckles are constructed as block copolymer micelles.EMBO J. 2021 Jun 15;40(12):e107270. doi: 10.15252/embj.2020107270. Epub 2021 Apr 22. EMBO J. 2021. PMID: 33885174 Free PMC article.
-
Molecular anatomy of the architectural NEAT1 noncoding RNA: The domains, interactors, and biogenesis pathway required to build phase-separated nuclear paraspeckles.Wiley Interdiscip Rev RNA. 2019 Nov;10(6):e1545. doi: 10.1002/wrna.1545. Epub 2019 May 1. Wiley Interdiscip Rev RNA. 2019. PMID: 31044562 Review.
-
Architectural RNAs for Membraneless Nuclear Body Formation.Cold Spring Harb Symp Quant Biol. 2019;84:227-237. doi: 10.1101/sqb.2019.84.039404. Epub 2020 Feb 4. Cold Spring Harb Symp Quant Biol. 2019. PMID: 32019862
-
Organization and function of paraspeckles.Essays Biochem. 2020 Dec 7;64(6):875-882. doi: 10.1042/EBC20200010. Essays Biochem. 2020. PMID: 32830222 Review.
Cited by
-
Exploration of the Noncoding Genome for Human-Specific Therapeutic Targets-Recent Insights at Molecular and Cellular Level.Cells. 2023 Nov 20;12(22):2660. doi: 10.3390/cells12222660. Cells. 2023. PMID: 37998395 Free PMC article. Review.
-
Dynamic Localization of Paraspeckle Components under Osmotic Stress.Noncoding RNA. 2024 Apr 12;10(2):23. doi: 10.3390/ncrna10020023. Noncoding RNA. 2024. PMID: 38668381 Free PMC article.
-
Improving the hole picture: towards a consensus on the mechanism of nuclear transport.Biochem Soc Trans. 2023 Apr 26;51(2):871-886. doi: 10.1042/BST20220494. Biochem Soc Trans. 2023. PMID: 37099395 Free PMC article.
-
Shell protein composition specified by the lncRNA NEAT1 domains dictates the formation of paraspeckles as distinct membraneless organelles.Nat Cell Biol. 2023 Nov;25(11):1664-1675. doi: 10.1038/s41556-023-01254-1. Epub 2023 Nov 6. Nat Cell Biol. 2023. PMID: 37932453
-
Biomolecules Interacting with Long Noncoding RNAs.Biology (Basel). 2025 Apr 19;14(4):442. doi: 10.3390/biology14040442. Biology (Basel). 2025. PMID: 40282307 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources

