Real-world efficacy and safety outcomes of imatinib treatment in patients with chronic myeloid leukemia: An Australian experience
- PMID: 36106342
- PMCID: PMC9475133
- DOI: 10.1002/prp2.1005
Real-world efficacy and safety outcomes of imatinib treatment in patients with chronic myeloid leukemia: An Australian experience
Abstract
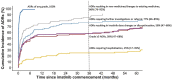
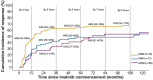
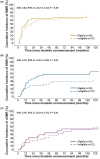
Tyrosine kinase inhibitors (TKI) have revolutionized the treatment of chronic myeloid leukemia (CML), but patients still experience treatment-limiting toxicities or therapeutic failure. To investigate the real-world use and outcomes of imatinib in patients with CML in Australia, a retrospective cohort study of patients with CML commencing imatinib (2001-2018) was conducted across two sites. Prescribing patterns, tolerability outcomes, and survival and molecular response were evaluated. 86 patients received 89 imatinib treatments. Dose modifications were frequently observed (12-month rate of 58%). At last follow-up, 62 patients (5-year rate of 55%) had permanently discontinued imatinib treatment, of which 44 switched to another TKI (5-year rate of 46%). Within 3 months of starting imatinib, 43% (95% CI, 32%-53%) of patients experienced imatinib-related grade ≥3 adverse drug reactions (ADRs). Higher comorbidity score, lower body weight, higher imatinib starting dose, and Middle Eastern or North African ancestry were associated with a higher risk of grade ≥3 ADR occurrence on multivariable analysis (MVA). Estimated overall survival and event-free survival rates at 3 years were 97% (95% CI, 92%-100%) and 81% (95% CI, 72%-92%), respectively. Cumulative incidence of major molecular response (MMR) at 3 years was 63% (95% CI, 50%-73%). On MVA, imatinib starting dose, ELTS score, BCR-ABL1 transcript type, pre-existing pulmonary disease, and potential drug-drug interactions were predictive of MMR. In conclusion, imatinib induced deep molecular responses that translated to good survival outcomes in a real-world setting, but was associated with a higher incidence of ADRs, dose modifications and treatment discontinuations than in clinical trials.
Keywords: anticancer drugs; chronic myeloid leukemia; imatinib; pharmacoepidemiology; precision medicine; real-world evidence; tyrosine kinase inhibitors.
© 2022 The Authors. Pharmacology Research & Perspectives published by British Pharmacological Society and American Society for Pharmacology and Experimental Therapeutics and John Wiley & Sons Ltd.
Figures




References
-
- Deininger M, O'Brien SG, Guilhot F, et al. International randomized study of interferon vs STI571 (IRIS) 8‐year follow up: sustained survival and low risk for progression or events in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML‐CP) treated with imatinib. Blood. 2009;114(22):1126. doi: 10.1182/blood.V114.22.1126.1126 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

