Dipolar pathways in multi-spin and multi-dimensional dipolar EPR spectroscopy
- PMID: 36106486
- PMCID: PMC9516884
- DOI: 10.1039/d2cp03048a
Dipolar pathways in multi-spin and multi-dimensional dipolar EPR spectroscopy
Abstract
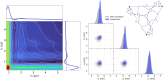
Dipolar electron paramagnetic resonance (EPR) experiments, such as double electron-electron resonance (DEER), measure distributions of nanometer-scale distances between unpaired electrons, which provide valuable information for structural characterization of proteins and other macromolecular systems. We present an extension to our previously published general model based on dipolar pathways valid for multi-dimensional dipolar EPR experiments with more than two spin-1/2 labels. We examine the 4-pulse DEER and TRIER experiments in terms of dipolar pathways and show experimental results confirming the theoretical predictions. This extension to the dipolar pathways model allows the analysis of previously challenging datasets and the extraction of multivariate distance distributions.
Conflict of interest statement
There are no conflicts to declare.
Figures












References
-
- Milov A. D. Salikhov K. M. Shchirov M. D. Soviet Phys. Solid State. 1981;23:565–569.
-
- Schiemann O. Heubach C. A. Abdullin D. Ackermann K. Azarkh M. Bagryanskaya E. G. Drescher M. Endeward B. Freed J. H. Galazzo L. Goldfarb D. Hett T. Esteban Hofer L. Fábregas Ibáñez L. Hustedt E. J. Kucher S. Kuprov I. Lovett J. E. Meyer A. Ruthstein S. Saxena S. Stoll S. Timmel C. R. Di Valentin M. Mchaourab H. S. Prisner T. F. Bode B. E. Bordignon E. Bennati M. Jeschke G. J. Am. Chem. Soc. 2021;143:17875–17890. - PMC - PubMed
-
- Schiemann O. Prisner T. F. Q. Rev. Biophys. 2007;40:1–53. - PubMed
-
- Jeschke G. Annu. Rev. Phys. Chem. 2012;63:419–446. - PubMed
-
- Drescher M., in EPR Spectroscopy: Applications in Chemistry and Biology, ed. M. Drescher and G. Jeschke, Springer, Berlin, Heidelberg, 2012, pp. 91–119
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

