The intestine is a major contributor to circulating succinate in mice
- PMID: 36106538
- PMCID: PMC9523828
- DOI: 10.1096/fj.202200135RR
The intestine is a major contributor to circulating succinate in mice
Abstract
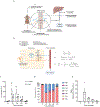
The tricarboxylic acid (TCA) cycle is the epicenter of cellular aerobic metabolism. TCA cycle intermediates facilitate energy production and provide anabolic precursors, but also function as intra- and extracellular metabolic signals regulating pleiotropic biological processes. Despite the importance of circulating TCA cycle metabolites as signaling molecules, the source of circulating TCA cycle intermediates remains uncertain. We observe that in mice, the concentration of TCA cycle intermediates in the portal blood exceeds that in tail blood indicating that the gut is a major contributor to circulating TCA cycle metabolites. With a focus on succinate as a representative of a TCA cycle intermediate with signaling activities and using a combination of gut microbiota depletion mouse models and isotopomer tracing, we demonstrate that intestinal microbiota is not a major contributor to circulating succinate. Moreover, we demonstrate that endogenous succinate production is markedly higher than intestinal succinate absorption in normal physiological conditions. Altogether, these results indicate that endogenous succinate production within the intestinal tissue is a major physiological source of circulating succinate. These results provide a foundation for an investigation into the role of the intestine in regulating circulating TCA cycle metabolites and their potential signaling effects on health and disease.
Keywords: TCA cycle intermediates; circulating biomarkers; intestine; succinate.
© 2022 Federation of American Societies for Experimental Biology.
Figures


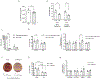




References
-
- Owen OE, Kalhan SC, and Hanson RW (2002) The key role of anaplerosis and cataplerosis for citric acid cycle function. The Journal of biological chemistry 277, 30409–30412 - PubMed
-
- He W, Miao FJ, Lin DC, Schwandner RT, Wang Z, Gao J, Chen JL, Tian H, and Ling L (2004) Citric acid cycle intermediates as ligands for orphan G-protein-coupled receptors. Nature 429, 188–193 - PubMed
-
- Lampropoulou V, Sergushichev A, Bambouskova M, Nair S, Vincent EE, Loginicheva E, Cervantes-Barragan L, Ma X, Huang SC, Griss T, Weinheimer CJ, Khader S, Randolph GJ, Pearce EJ, Jones RG, Diwan A, Diamond MS, and Artyomov MN (2016) Itaconate Links Inhibition of Succinate Dehydrogenase with Macrophage Metabolic Remodeling and Regulation of Inflammation. Cell metabolism 24, 158–166 - PMC - PubMed
-
- Rubic T, Lametschwandtner G, Jost S, Hinteregger S, Kund J, Carballido-Perrig N, Schwärzler C, Junt T, Voshol H, Meingassner JG, Mao X, Werner G, Rot A, and Carballido JM (2008) Triggering the succinate receptor GPR91 on dendritic cells enhances immunity. Nature immunology 9, 1261–1269 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources

