Durability of protection and immunogenicity of AZD1222 (ChAdOx1 nCoV-19) COVID-19 vaccine over 6 months
- PMID: 36106642
- PMCID: PMC9479753
- DOI: 10.1172/JCI160565
Durability of protection and immunogenicity of AZD1222 (ChAdOx1 nCoV-19) COVID-19 vaccine over 6 months
Abstract
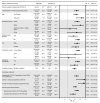
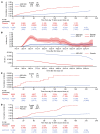
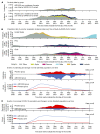
BackgroundWe report updated safety, efficacy, and immunogenicity of AZD1222 (ChAdOx1 nCoV-19) from an ongoing phase 3 trial.MethodsAdults at increased risk of SARS-CoV-2 infection were randomized (2:1), stratified by age, to receive 2 doses of AZD1222 or placebo. The primary efficacy end point was confirmed SARS-CoV-2 reverse-transcriptase PCR-positive (RT-PCR-positive) symptomatic COVID-19 at 15 or more days after a second dose in baseline SARS-CoV-2-seronegative participants. The 21,634 and 10,816 participants were randomized to AZD1222 and placebo, respectively.FindingsData cutoff for this analysis was July 30, 2021; median follow-up from second dose was 78 and 71 days for the double-blind period (censoring at unblinding or nonstudy COVID-19 vaccination) and 201 and 82 days for the period to nonstudy COVID-19 vaccination (regardless of unblinding) in the AZD1222 and placebo groups, respectively. For the primary efficacy end point in the double-blind period (141 and 184 events; incidence rates: 39.2 and 118.8 per 1,000 person years), vaccine efficacy was 67.0% (P < 0.001). In the period to nonstudy COVID-19 vaccination, incidence of events remained consistently low and stable through 6 months in the AZD1222 group; for the primary efficacy end point (328 and 219 events; incidence rates: 36.4, 108.4) and severe/critical disease (5 and 13 events; incidence rates: 0.6, 6.4), respective vaccine efficacy estimates were 65.1% and 92.1%. AZD1222 elicited humoral immune responses over time, with waning at day 180. No emergent safety issues were seen.ConclusionAZD1222 is safe and well tolerated, demonstrating durable protection and immunogenicity with median follow-up (AZD1222 group) of 6 months.Trial registrationClinicalTrials.gov NCT04516746.FundingAstraZeneca; US government.
Keywords: Adaptive immunity; COVID-19.
Figures



Comment in
-
Lessons from a large-scale COVID-19 vaccine trial.J Clin Invest. 2022 Sep 15;132(18):e163202. doi: 10.1172/JCI163202. J Clin Invest. 2022. PMID: 36106630 Free PMC article.
References
-
- CDC. COVID Data Tracker. https://covid.cdc.gov/covid-data-tracker/#datatracker-home. Updated July 5, 2022. Accessed March 9, 2022.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

