Mortality and Morbidity Effects of Long-Term Exposure to Low-Level PM2.5, BC, NO2, and O3: An Analysis of European Cohorts in the ELAPSE Project
- PMID: 36106702
- PMCID: PMC9476567
Mortality and Morbidity Effects of Long-Term Exposure to Low-Level PM2.5, BC, NO2, and O3: An Analysis of European Cohorts in the ELAPSE Project
Abstract
Introduction: Epidemiological cohort studies have consistently found associations between long-term exposure to outdoor air pollution and a range of morbidity and mortality endpoints. Recent evaluations by the World Health Organization and the Global Burden of Disease study have suggested that these associations may be nonlinear and may persist at very low concentrations. Studies conducted in North America in particular have suggested that associations with mortality persisted at concentrations of particulate matter with an aerodynamic diameter of less than 2.5 μm (PM2.5) well below current air quality standards and guidelines. The uncertainty about the shape of the concentration-response function at the low end of the concentration distribution, related to the scarcity of observations in the lowest range, was the basis of the current project. Previous studies have focused on PM2.5, but increasingly associations with nitrogen dioxide (NO2) are being reported, particularly in studies that accounted for the fine spatial scale variation of NO2. Very few studies have evaluated the effects of long-term exposure to low concentrations of ozone (O3). Health effects of black carbon (BC), representing primary combustion particles, have not been studied in most large cohort studies of PM2.5. Cohort studies assessing health effects of particle composition, including elements from nontailpipe traffic emissions (iron, copper, and zinc) and secondary aerosol (sulfur) have been few in number and reported inconsistent results. The overall objective of our study was to investigate the shape of the relationship between long-term exposure to four pollutants (PM2.5, NO2, BC, and O3) and four broad health effect categories using a number of different methods to characterize the concentration-response function (i.e., linear, nonlinear, or threshold). The four health effect categories were (1) natural- and cause-specific mortality including cardiovascular and nonmalignant as well as malignant respiratory and diabetes mortality; and morbidity measured as (2) coronary and cerebrovascular events; (3) lung cancer incidence; and (4) asthma and chronic obstructive pulmonary disease (COPD) incidence. We additionally assessed health effects of PM2.5 composition, specifically the copper, iron, zinc, and sulfur content of PM2,5.
Methods: We focused on analyses of health effects of air pollutants at low concentrations, defined as less than current European Union (EU) Limit Values, U.S. Environmental Protection Agency (U.S. EPA), National Ambient Air Quality Standards (NAAQS), and/or World Health Organization (WHO) Air Quality Guideline values for PM2.5, NO2, and O3. We address the health effects at low air pollution levels by performing new analyses within selected cohorts of the ESCAPE study (European Study of Cohorts for Air Pollution Effects; Beelen et al. 2014a) and within seven very large European administrative cohorts. By combining well-characterized ESCAPE cohorts and large administrative cohorts in one study the strengths and weaknesses of each approach can be addressed. The large administrative cohorts are more representative of national or citywide populations, have higher statistical power, and can efficiently control for area-level confounders, but have fewer possibilities to control for individual-level confounders. The ESCAPE cohorts have detailed information on individual confounders, as well as country-specific information on area-level confounding. The data from the seven included ESCAPE cohorts and one additional non-ESCAPE cohort have been pooled and analyzed centrally. More than 300,000 adults were included in the pooled cohort from existing cohorts in Sweden, Denmark, Germany, the Netherlands, Austria, France, and Italy. Data from the administrative cohorts have been analyzed locally, without transfer to a central database. Privacy regulations prevented transfer of data from administrative cohorts to a central database. More than 28 million adults were included from national administrative cohorts in Belgium, Denmark, England, the Netherlands, Norway, and Switzerland as well as an administrative cohort in Rome, Italy. We developed central exposure assessment using Europewide hybrid land use regression (LUR) models, which incorporated European routine monitoring data for PM2.5, NO2, and O3, and ESCAPE monitoring data for BC and PM2.5 composition, land use, and traffic data supplemented with satellite observations and chemical transport model estimates. For all pollutants, we assessed exposure at a fine spatial scale, 100 × 100 m grids. These models have been applied to individual addresses of all cohorts including the administrative cohorts. In sensitivity analyses, we applied the PM2.5 models developed within the companion HEI-funded Canadian MAPLE study (Brauer et al. 2019) and O3 exposures on a larger spatial scale for comparison with previous studies. Identification of outcomes included linkage with mortality, cancer incidence, hospital discharge registries, and physician-based adjudication of cases. We analyzed natural-cause, cardiovascular, ischemic heart disease, stroke, diabetes, cardiometabolic, respiratory, and COPD mortality. We also analyzed lung cancer incidence, incidence of coronary and cerebrovascular events, and incidence of asthma and COPD (pooled cohort only). We applied the Cox proportional hazard model with increasing control for individual- and area-level covariates to analyze the associations between air pollution and mortality and/or morbidity for both the pooled cohort and the individual administrative cohorts. Age was used as the timescale because of evidence that this results in better adjustment for potential confounding by age. Censoring occurred at the time of the event of interest, death from other causes, emigration, loss to follow-up for other reasons, or at the end of follow-up, whichever came first. A priori we specified three confounder models, following the modeling methods of the ESCAPE study. Model 1 included only age (time axis), sex (as strata), and calendar year of enrollment. Model 2 added individual-level variables that were consistently available in the cohorts contributing to the pooled cohort or all variables available in the administrative cohorts, respectively. Model 3 further added area-level socioeconomic status (SES) variables. A priori model 3 was selected as the main model. All analyses in the pooled cohort were stratified by subcohort. All analyses in the administrative cohorts accounted for clustering of the data in neighborhoods by adjusting the variance of the effect estimates. The main exposure variable we analyzed was derived from the Europewide hybrid models based on 2010 monitoring data. Sensitivity analyses were conducted using earlier time periods, time-varying exposure analyses, local exposure models, and the PM2.5 models from the Canadian MAPLE project. We first specified linear single-pollutant models. Two-pollutant models were specified for all combinations of the four main pollutants. Two-pollutant models for particle composition were analyzed with PM2.5 and NO2 as the second pollutant. We then investigated the shape of the concentration-response function using natural splines with two, three, and four degrees of freedom; penalized splines with the degrees of freedom determined by the algorithm and shape-constrained health impact functions (SCHIF) using confounder model 3. Additionally, we specified linear models in subsets of the concentration range, defined by removing concentrations above a certain value from the analysis, such as for PM2.5 25 μg/m3 (EU limit value), 20, 15, 12 μg/m3 (U.S. EPA National Ambient Air Quality Standard), and 10 μg/m3 (WHO Air Quality Guideline value). Finally, threshold models were evaluated to investigate whether the associations persisted below specific concentration values. For PM2.5, we evaluated 10, 7.5, and 5 μg/m3 as potential thresholds. Performance of threshold models versus the corresponding no-threshold linear model were evaluated using the Akaike information criterion (AIC).
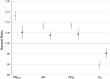
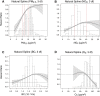
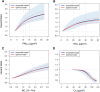
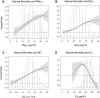
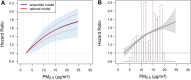
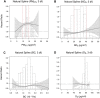
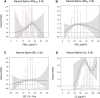
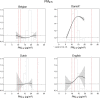
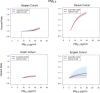
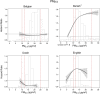
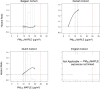
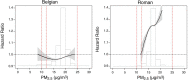
Results: In the pooled cohort, virtually all subjects in 2010 had PM2.5 and NO2 annual average exposures below the EU limit values (25 μg/m3 and 40 μg/m3, respectively). More than 50,000 had a residential PM2.5 exposure below the U.S. EPA NAAQS (12 μg/m3). More than 25,000 subjects had a residential PM2.5 exposure below the WHO guideline (10 μg/m3). We found significant positive associations between PM2.5, NO2, and BC and natural-cause, respiratory, cardiovascular, and diabetes mortality. In our main model, the hazard ratios (HRs) (95% [confidence interval] CI) were 1.13 (CI = 1.11, 1.16) for an increase of 5 μg/m3 PM2.5, 1.09 (CI = 1.07, 1.10) for an increase of 10 μg/m3 NO2, and 1.08 (CI = 1.06, 1.10) for an increase of 0.5 × 10-5/m BC for natural-cause mortality. The highest HRs were found for diabetes mortality. Associations with O3 were negative, both in the fine spatial scale of the main ELAPSE model and in large spatial scale exposure models. For PM2.5, NO2, and BC, we generally observed a supralinear association with steeper slopes at low exposures and no evidence of a concentration below which no association was found. Subset analyses further confirmed that these associations remained at low levels: below 10 μg/m3 for PM2.5 and 20 μg/m3 for NO2. HRs were similar to the full cohort HRs for subjects with exposures below the EU limit values for PM2.5 and NO2, the U.S. NAAQS values for PM2.5, and the WHO guidelines for PM2.5 and NO2. The mortality associations were robust to alternative specifications of exposure, including different time periods, PM2.5 from the MAPLE project, and estimates from the local ESCAPE model. Time-varying exposure natural spline analyses confirmed associations at low pollution levels. HRs in two-pollutant models were attenuated but remained elevated and statistically significant for PM2.5 and NO2. In two-pollutant models of PM2.5 and NO2 HRs for natural-cause mortality were 1.08 (CI = 1.05, 1.11) for PM2.5 and 1.05 (CI = 1.03, 1.07) for NO2. Associations with O3 were attenuated but remained negative in two-pollutant models with NO2, BC, and PM2.5. We found significant positive associations between PM2.5, NO2, and BC and incidence of stroke and asthma and COPD hospital admissions. Furthermore, NO2 was significantly related to acute coronary heart disease and PM2.5 was significantly related to lung cancer incidence. We generally observed linear to supralinear associations with no evidence of a threshold, with the exception of the association between NO2 and acute coronary heart disease, which was sublinear. Subset analyses documented that associations remained even with PM2.5 below 20 μg/m3 and possibly 12 μg/m3. Associations remained even when NO2 was below 30 μg/m3 and in some cases 20 μg/m3. In two-pollutant models, NO2 was most consistently associated with acute coronary heart disease, stroke, asthma, and COPD hospital admissions. PM2.5 was not associated with these outcomes in two-pollutant models with NO2. PM2.5 was the only pollutant that was associated with lung cancer incidence in two-pollutant models. Associations with O3 were negative though generally not statistically significant. In the administrative cohorts, virtually all subjects in 2010 had PM2.5 and NO2 annual average exposures below the EU limit values. More than 3.9 million subjects had a residential PM2.5 exposure below the U.S. EPA NAAQS (12 μg/m3) and more than 1.9 million had residential PM2.5 exposures below the WHO guideline (10 μg/m3). We found significant positive associations between PM2.5, NO2, and BC and natural-cause, respiratory, cardiovascular, and lung cancer mortality, with moderate to high heterogeneity between cohorts. We found positive but statistically nonsignificant associations with diabetes mortality. In our main model meta-analysis, the HRs (95% CI) for natural-cause mortality were 1.05 (CI = 1.02, 1.09) for an increase of 5 μg/m3 PM2.5, 1.04 (CI = 1.02, 1.07) for an increase of 10 μg/m3 NO2, and 1.04 (CI = 1.02, 1.06) for an increase of 0.5 × 10-5/m BC, and 0.95 (CI = 0.93, 0.98) for an increase of 10 μg/m3 O3. The shape of the concentration-response functions differed between cohorts, though the associations were generally linear to supralinear, with no indication of a level below which no associations were found. Subset analyses documented that these associations remained at low levels: below 10 μg/m3 for PM2.5 and 20 μg/m3 for NO2. BC and NO2 remained significantly associated with mortality in two-pollutant models with PM2.5 and O3. The PM2.5 HR attenuated to unity in a two-pollutant model with NO2. The negative O3 association was attenuated to unity and became nonsignificant. The mortality associations were robust to alternative specifications of exposure, including time-varying exposure analyses. Time-varying exposure natural spline analyses confirmed associations at low pollution levels. Effect estimates in the youngest participants (<65 years at baseline) were much larger than in the elderly (>65 years at baseline). Effect estimates obtained with the ELAPSE PM2.5 model did not differ from the MAPLE PM2.5 model on average, but in individual cohorts, substantial differences were found.
Conclusions: Long-term exposure to PM2.5, NO2, and BC was positively associated with natural-cause and cause-specific mortality in the pooled cohort and the administrative cohorts. Associations were found well below current limit values and guidelines for PM2.5 and NO2. Associations tended to be supralinear, with steeper slopes at low exposures with no indication of a threshold. Two-pollutant models documented the importance of characterizing the ambient mixture with both NO2 and PM2.5. We mostly found negative associations with O3. In two-pollutant models with NO2, the negative associations with O3 were attenuated to essentially unity in the mortality analysis of the administrative cohorts and the incidence analyses in the pooled cohort. In the mortality analysis of the pooled cohort, significant negative associations with O3 remained in two-pollutant models. Long-term exposure to PM2.5, NO2, and BC was also positively associated with morbidity outcomes in the pooled cohort. For stroke, asthma, and COPD, positive associations were found for PM2.5, NO2, and BC. For acute coronary heart disease, an increased HR was observed for NO2. For lung cancer, an increased HR was found only for PM2.5. Associations mostly showed steeper slopes at low exposures with no indication of a threshold.
© 2021 Health Effects Institute. All rights reserved.
Figures























References
-
- Andersen ZJ, Hvidberg M, Jensen SS, Ketzel M, Loft S, Sørensen M, et al. . 2011. Chronic obstructive pulmonary disease and long-term exposure to traffic-related air pollution: A cohort study. Am J Respir Crit Care Med 183:455–461. - PubMed
-
- Atkinson RW, Carey IM, Kent AJ, van Staa TP, Anderson HR, Cook DG. 2013. Long-term exposure to outdoor air pollution and incidence of cardiovascular diseases. Epidemiology 24:44–53. - PubMed
-
- Bai L, Shin S, Burnett RT, Kwong JC, Hystad P, van Donkelaar A, et al. . 2020. Exposure to ambient air pollution and the incidence of lung cancer and breast cancer in the Ontario population health and environment cohort. Int J Cancer 146:2450–2459. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
