Conformational transitions of the Spindly adaptor underlie its interaction with Dynein and Dynactin
- PMID: 36107127
- PMCID: PMC9481740
- DOI: 10.1083/jcb.202206131
Conformational transitions of the Spindly adaptor underlie its interaction with Dynein and Dynactin
Abstract
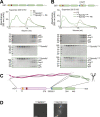
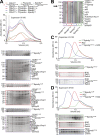
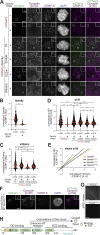
Cytoplasmic Dynein 1, or Dynein, is a microtubule minus end-directed motor. Dynein motility requires Dynactin and a family of activating adaptors that stabilize the Dynein-Dynactin complex and promote regulated interactions with cargo in space and time. How activating adaptors limit Dynein activation to specialized subcellular locales is unclear. Here, we reveal that Spindly, a mitotic Dynein adaptor at the kinetochore corona, exists natively in a closed conformation that occludes binding of Dynein-Dynactin to its CC1 box and Spindly motif. A structure-based analysis identified various mutations promoting an open conformation of Spindly that binds Dynein-Dynactin. A region of Spindly downstream from the Spindly motif and not required for cargo binding faces the CC1 box and stabilizes the intramolecular closed conformation. This region is also required for robust kinetochore localization of Spindly, suggesting that kinetochores promote Spindly activation to recruit Dynein. Thus, our work illustrates how specific Dynein activation at a defined cellular locale may require multiple factors.
© 2022 d'Amico et al.
Figures











Comment in
-
SPIN(DLY)-OFF: A tale of conformational change to control DYNEIN.J Cell Biol. 2022 Nov 7;221(11):e202209063. doi: 10.1083/jcb.202209063. Epub 2022 Oct 6. J Cell Biol. 2022. PMID: 36200976 Free PMC article.
References
-
- Alex, A., Piano V., Polley S., Stuiver M., Voss S., Ciossani G., Overlack K., Voss B., Wohlgemuth S., Petrovic A., et al. . 2019. Electroporated recombinant proteins as tools for in vivo functional complementation, imaging and chemical biology. Elife. 8:e48287. 10.7554/eLife.48287 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

