Impact of integrating genomic data into the electronic health record on genetics care delivery
- PMID: 36107166
- PMCID: PMC10176082
- DOI: 10.1016/j.gim.2022.08.009
Impact of integrating genomic data into the electronic health record on genetics care delivery
Abstract
Purpose: Integrating genomic data into the electronic health record (EHR) is key for optimally delivering genomic medicine.
Methods: The PennChart Genomics Initiative (PGI) at the University of Pennsylvania is a multidisciplinary collaborative that has successfully linked orders and results from genetic testing laboratories with discrete genetic data in the EHR. We quantified the use of the genomic data within the EHR, performed a time study with genetic counselors, and conducted key informant interviews with PGI members to evaluate the effect of the PGI's efforts on genetics care delivery.
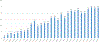
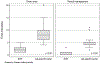
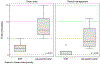
Results: The PGI has interfaced with 4 genetic testing laboratories, resulting in the creation of 420 unique computerized genetic testing orders that have been used 4073 times to date. In a time study of 96 genetic testing activities, EHR use was associated with significant reductions in time spent ordering (2 vs 8 minutes, P < .001) and managing (1 vs 5 minutes, P < .001) genetic results compared with the use of online laboratory-specific portals. In key informant interviews, multidisciplinary collaboration and institutional buy-in were identified as key ingredients for the PGI's success.
Conclusion: The PGI's efforts to integrate genomic medicine into the EHR have substantially streamlined the delivery of genomic medicine.
Keywords: Electronic health record; Genomics; Integration.
Copyright © 2022 American College of Medical Genetics and Genomics. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest K.S.L.-M. has an immediate family member who is employed by GlaxoSmithKline. All other authors declare no conflict of interest.
Figures






References
-
- National Comprehensive Cancer Network. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic. 2020.
-
- National Comprehensive Cancer Network. Genetic/Familial High-Risk Assessment: Colorectal. 2020.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

