Elevating microglia TREM2 reduces amyloid seeding and suppresses disease-associated microglia
- PMID: 36107206
- PMCID: PMC9481739
- DOI: 10.1084/jem.20212479
Elevating microglia TREM2 reduces amyloid seeding and suppresses disease-associated microglia
Abstract
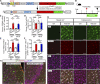
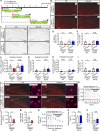
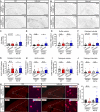
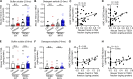
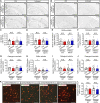
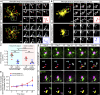
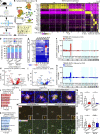
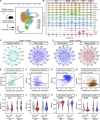
TREM2 is exclusively expressed by microglia in the brain and is strongly linked to the risk for Alzheimer's disease (AD). As microglial responses modulated by TREM2 are central to AD pathogenesis, enhancing TREM2 signaling has been explored as an AD therapeutic strategy. However, the effective therapeutic window targeting TREM2 is unclear. Here, by using microglia-specific inducible mouse models overexpressing human wild-type TREM2 (TREM2-WT) or R47H risk variant (TREM2-R47H), we show that TREM2-WT expression reduces amyloid deposition and neuritic dystrophy only during the early amyloid seeding stage, whereas TREM2-R47H exacerbates amyloid burden during the middle amyloid rapid growth stage. Single-cell RNA sequencing reveals suppressed disease-associated microglia (DAM) signature and reduced DAM population upon TREM2-WT expression in the early stage, whereas upregulated antigen presentation pathway is detected with TREM2-R47H expression in the middle stage. Together, our findings highlight the dynamic effects of TREM2 in modulating AD pathogenesis and emphasize the beneficial effect of enhancing TREM2 function in the early stage of AD development.
© 2022 Zhao et al.
Conflict of interest statement
Disclosures: G. Bu reported grants from National Institutes of Health during the conduct of the study; personal fees from SciNeuro, Kisbee, and Lexeo outside the submitted work. No other disclosures were reported.
Figures





References
-
- Bemiller, S.M., McCray T.J., Allan K., Formica S.V., Xu G., Wilson G., Kokiko-Cochran O.N., Crish S.D., Lasagna-Reeves C.A., Ransohoff R.M., et al. . 2017. TREM2 deficiency exacerbates tau pathology through dysregulated kinase signaling in a mouse model of tauopathy. Mol. Neurodegener. 12:74. 10.1186/s13024-017-0216-6 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

