A neurogenic signature involving monoamine Oxidase-A controls human thermogenic adipose tissue development
- PMID: 36107478
- PMCID: PMC9519151
- DOI: 10.7554/eLife.78945
A neurogenic signature involving monoamine Oxidase-A controls human thermogenic adipose tissue development
Abstract
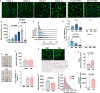
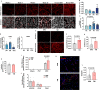
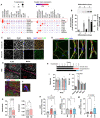
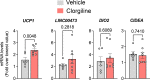
Mechanisms that control 'beige/brite' thermogenic adipose tissue development may be harnessed to improve human metabolic health. To define these mechanisms, we developed a species-hybrid model in which human mesenchymal progenitor cells were used to develop white or thermogenic/beige adipose tissue in mice. The hybrid adipose tissue developed distinctive features of human adipose tissue, such as larger adipocyte size, despite its neurovascular architecture being entirely of murine origin. Thermogenic adipose tissue recruited a denser, qualitatively distinct vascular network, differing in genes mapping to circadian rhythm pathways, and denser sympathetic innervation. The enhanced thermogenic neurovascular network was associated with human adipocyte expression of THBS4, TNC, NTRK3, and SPARCL1, which enhance neurogenesis, and decreased expression of MAOA and ACHE, which control neurotransmitter tone. Systemic inhibition of MAOA, which is present in human but absent in mouse adipocytes, induced browning of human but not mouse adipose tissue, revealing the physiological relevance of this pathway. Our results reveal species-specific cell type dependencies controlling the development of thermogenic adipose tissue and point to human adipocyte MAOA as a potential target for metabolic disease therapy.
Keywords: developmental biology; diabetes; human; metabolism; mouse; obesity.
© 2022, Solivan-Rivera et al.
Conflict of interest statement
JS, ZY, TD, AD, SP, QY, RR, RZ, PS, SJ, DZ, TN, SC No competing interests declared
Figures





Similar articles
-
Different action of glucocorticoid receptor in adipose tissue remodelling to modulate energy homeostasis by chronic restraint stress.Lipids Health Dis. 2025 Mar 27;24(1):121. doi: 10.1186/s12944-025-02539-0. Lipids Health Dis. 2025. PMID: 40148860 Free PMC article.
-
S100A8/S100A9 impairs energy expenditure and whole body metabolism.Am J Physiol Endocrinol Metab. 2025 Aug 1;329(2):E341-E354. doi: 10.1152/ajpendo.00076.2025. Epub 2025 Jul 15. Am J Physiol Endocrinol Metab. 2025. PMID: 40662569
-
Exosome-Derived miR-11987 in Bovine Milk Inhibits Obesity Through Browning of White Fat.Int J Mol Sci. 2025 Jun 23;26(13):6006. doi: 10.3390/ijms26136006. Int J Mol Sci. 2025. PMID: 40649784 Free PMC article.
-
Adipose tissue browning and thermogenesis under physiologically energetic challenges: a remodelled thermogenic system.J Physiol. 2024 Jan;602(1):23-48. doi: 10.1113/JP285269. Epub 2023 Nov 29. J Physiol. 2024. PMID: 38019069 Review.
-
Molecular epigenetics in the transition of white to brown fat.Pathol Res Pract. 2025 Aug;272:156073. doi: 10.1016/j.prp.2025.156073. Epub 2025 Jun 4. Pathol Res Pract. 2025. PMID: 40482385 Review.
Cited by
-
Increased OCT3 Expression in Adipose Tissue With Aging: Implications for Catecholamine and Lipid Turnover and Insulin Resistance in Women.Endocrinology. 2023 Nov 20;165(1):bqad172. doi: 10.1210/endocr/bqad172. Endocrinology. 2023. PMID: 37972266 Free PMC article.
-
Association between personality traits, eating behaviors, and the genetic polymorphisms FTO-rs9939609 and MAO-A 30 bp u-VNTR with obesity in Mexican Mayan children.Front Genet. 2024 Jul 26;15:1421870. doi: 10.3389/fgene.2024.1421870. eCollection 2024. Front Genet. 2024. PMID: 39130748 Free PMC article.
-
Involution of brown adipose tissue through a Syntaxin 4 dependent pyroptosis pathway.Nat Commun. 2024 Apr 2;15(1):2856. doi: 10.1038/s41467-024-46944-y. Nat Commun. 2024. PMID: 38565851 Free PMC article.
-
Wnt signaling preserves progenitor cell multipotency during adipose tissue development.Nat Metab. 2023 Jun;5(6):1014-1028. doi: 10.1038/s42255-023-00813-y. Epub 2023 Jun 19. Nat Metab. 2023. PMID: 37337125 Free PMC article.
-
Adipose tissue as a linchpin of organismal ageing.Nat Metab. 2024 May;6(5):793-807. doi: 10.1038/s42255-024-01046-3. Epub 2024 May 23. Nat Metab. 2024. PMID: 38783156 Free PMC article. Review.
References
-
- Aran D, Looney AP, Liu L, Wu E, Fong V, Hsu A, Chak S, Naikawadi RP, Wolters PJ, Abate AR, Butte AJ, Bhattacharya M. Reference-Based analysis of lung single-cell sequencing reveals a transitional profibrotic macrophage. Nature Immunology. 2019;20:163–172. doi: 10.1038/s41590-018-0276-y. - DOI - PMC - PubMed
-
- Bour S, Daviaud D, Gres S, Lefort C, Prévot D, Zorzano A, Wabitsch M, Saulnier-Blache J-S, Valet P, Carpéné C. Adipogenesis-Related increase of semicarbazide-sensitive amine oxidase and monoamine oxidase in human adipocytes. Biochimie. 2007;89:916–925. doi: 10.1016/j.biochi.2007.02.013. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- SRA/SRX4074089
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

