Individualizing the Oncological Treatment of Patients With Metastatic Non-Clear Cell Renal Cell Carcinoma by Using Gene Sequencing and Patient-Reported Outcomes: Protocol for the INDIGO Study
- PMID: 36107483
- PMCID: PMC9523525
- DOI: 10.2196/36632
Individualizing the Oncological Treatment of Patients With Metastatic Non-Clear Cell Renal Cell Carcinoma by Using Gene Sequencing and Patient-Reported Outcomes: Protocol for the INDIGO Study
Abstract
Background: No phase 3 studies have yet been conducted for patients with non-clear cell (CC) renal cell carcinoma (RCC) exclusively due to the rare occurrence of the disease and the heterogenicity in tumor morphology. Consequently, there is no evidence of the optimal treatment, and new approaches are needed. One approach is individualizing treatment based on the gene sequencing of tumor tissue. Additionally, recent studies involving the patient-reported outcomes (PROs) of patients treated for metastatic cancer have shown significant benefits for quality of life, median overall survival, and overall survival. The use of gene sequencing and PROs can be of great importance to patients with rare cancer types, including patients with non-CC RCC, and should be investigated in clinical trials, especially for cases where evidence based on phase 3 studies is difficult to obtain.
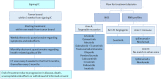
Objective: We describe the INDIGO study, in which patients, based on gene analyses, will be allocated into 4 treatment arms containing 14 treatments and use electronic PROs. We aim to improve the treatment of patients with non-CC RCC. The end points in the study will be the overall response rate (complete and partial) in the total patient population, which will be based on the RECIST (Response Evaluation Criteria in Solid Tumors) version 1.1 criteria, and the time to treatment failure.
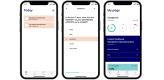
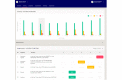
Methods: INDIGO is a prospective phase 2 trial, and 30 patients will be enrolled. The patients will receive systemic treatment based on genetic analyses of their tumor tissue. All patients will receive electronic questionnaires in a dedicated app-a questionnaire regarding symptoms and side effects and another regarding health-related quality of life. Depending on the treatment regimen, the patients will be seen by a medical doctor every third, fourth, or sixth week, and the effect of the systemic treatment will be evaluated every 6 weeks via a computed tomography scan. The study has been approved by the Danish Medicines Agency and the National Committee on Health Research Ethics (approval number: H-19041833), complies with good clinical practice guidelines, follows the General Data Protection Regulation, and is registered at the Capital Region of Denmark.
Results: Recruitment started in March 2020, and at the time of submitting this paper (June 2022), a total of 9 patients have been enrolled.
Conclusions: We aim to explore methods for improving the treatment outcomes of patients with non-CC RCC, and the INDIGO study will contribute further data on personalized medicine for rare types of RCC and provide new knowledge on the active use of electronic PROs.
Trial registration: ClinicalTrials.gov NCT04644432, https://clinicaltrials.gov/ct2/show/NCT04644432 ; European Union Drug Regulating Authorities Clinical Trials Database 2019-001316-38, https://tinyurl.com/2p8mb4aw.
International registered report identifier (irrid): DERR1-10.2196/36632.
Keywords: eHealth; electronic patient-reported outcome; health-related quality of life; non–clear cell renal cell carcinoma; oncology; outcome; patient-reported; patient-reported outcome; precision medicine; renal cell carcinoma; targeted therapy.
©Ida Marie Lind Rasmussen, Anne Vest Soerensen, Anne Kirstine Møller, Gitte Fredberg Persson, Jesper Andreas Palshof, Gry Assam Taarnhøj, Helle Pappot. Originally published in JMIR Research Protocols (https://www.researchprotocols.org), 15.09.2022.
Conflict of interest statement
Conflicts of Interest: The INDIGO study is financially supported by Roche Pharmaceuticals and The Capital Region of Denmark. None of the authors are funded by Roche Pharmaceuticals.
Figures
References
-
- Escudier B, Porta C, Schmidinger M, Rioux-Leclercq N, Bex A, Khoo V, Grünwald V, Gillessen S, Horwich A, ESMO Guidelines Committee Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol. 2019 May 01;30(5):706–720. doi: 10.1093/annonc/mdz056. https://linkinghub.elsevier.com/retrieve/pii/S0923-7534(19)31157-3 S0923-7534(19)31157-3 - DOI - PubMed
-
- Kroeger N, Xie W, Lee JL, Bjarnason GA, Knox JJ, Mackenzie MJ, Wood L, Srinivas S, Vaishamayan UN, Rha SY, Pal SK, Yuasa T, Donskov F, Agarwal N, Kollmannsberger CK, Tan MH, North SA, Rini BI, Choueiri TK, Heng DYC. Metastatic non-clear cell renal cell carcinoma treated with targeted therapy agents: characterization of survival outcome and application of the International mRCC Database Consortium criteria. Cancer. 2013 Aug 15;119(16):2999–3006. doi: 10.1002/cncr.28151. doi: 10.1002/cncr.28151. - DOI - DOI - PMC - PubMed
-
- Armstrong AJ, Halabi S, Eisen T, Broderick S, Stadler WM, Jones RJ, Garcia JA, Vaishampayan UN, Picus J, Hawkins RE, Hainsworth JD, Kollmannsberger CK, Logan TF, Puzanov I, Pickering LM, Ryan CW, Protheroe A, Lusk CM, Oberg S, George DJ. Everolimus versus sunitinib for patients with metastatic non-clear cell renal cell carcinoma (ASPEN): a multicentre, open-label, randomised phase 2 trial. Lancet Oncol. 2016 Mar;17(3):378–388. doi: 10.1016/S1470-2045(15)00515-X. http://europepmc.org/abstract/MED/26794930 S1470-2045(15)00515-X - DOI - PMC - PubMed
-
- Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, Grünwald V, Thompson JA, Figlin RA, Hollaender N, Urbanowitz G, Berg WJ, Kay A, Lebwohl D, Ravaud A, RECORD-1 Study Group Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008 Aug 09;372(9637):449–456. doi: 10.1016/S0140-6736(08)61039-9.S0140-6736(08)61039-9 - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

