Tracking of progressing human DNA polymerase δ holoenzymes reveals distributions of DNA lesion bypass activities
- PMID: 36107777
- PMCID: PMC9508823
- DOI: 10.1093/nar/gkac745
Tracking of progressing human DNA polymerase δ holoenzymes reveals distributions of DNA lesion bypass activities
Abstract
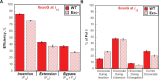
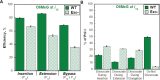
During DNA replication, DNA lesions in lagging strand templates are initially encountered by DNA polymerase δ (pol δ) holoenzymes comprised of pol δ and the PCNA processivity sliding clamp. These encounters are thought to stall replication of an afflicted template before the lesion, activating DNA damage tolerance (DDT) pathways that replicate the lesion and adjacent DNA sequence, allowing pol δ to resume. However, qualitative studies observed that human pol δ can replicate various DNA lesions, albeit with unknown proficiencies, which raises issues regarding the role of DDT in replicating DNA lesions. To address these issues, we re-constituted human lagging strand replication to quantitatively characterize initial encounters of pol δ holoenzymes with DNA lesions. The results indicate pol δ holoenzymes support dNTP incorporation opposite and beyond multiple lesions and the extent of these activities depends on the lesion and pol δ proofreading. Furthermore, after encountering a given DNA lesion, subsequent dissociation of pol δ is distributed around the lesion and a portion does not dissociate. The distributions of these events are dependent on the lesion and pol δ proofreading. Collectively, these results reveal complexity and heterogeneity in the replication of lagging strand DNA lesions, significantly advancing our understanding of human DDT.
© The Author(s) 2022. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures









Similar articles
-
A human high-fidelity DNA polymerase holoenzyme has a wide range of lesion bypass activities.Nucleic Acids Res. 2025 Jul 8;53(13):gkaf646. doi: 10.1093/nar/gkaf646. Nucleic Acids Res. 2025. PMID: 40671520 Free PMC article.
-
A human high-fidelity DNA polymerase holoenzyme has a wide range of lesion bypass activities.bioRxiv [Preprint]. 2024 Oct 17:2024.10.14.618244. doi: 10.1101/2024.10.14.618244. bioRxiv. 2024. Update in: Nucleic Acids Res. 2025 Jul 8;53(13):gkaf646. doi: 10.1093/nar/gkaf646. PMID: 39464047 Free PMC article. Updated. Preprint.
-
Replication protein A dynamically re-organizes on primer/template junctions to permit DNA polymerase δ holoenzyme assembly and initiation of DNA synthesis.Nucleic Acids Res. 2024 Jul 22;52(13):7650-7664. doi: 10.1093/nar/gkae475. Nucleic Acids Res. 2024. PMID: 38842913 Free PMC article.
-
Eukaryotic DNA polymerase ζ.DNA Repair (Amst). 2015 May;29:47-55. doi: 10.1016/j.dnarep.2015.02.012. Epub 2015 Feb 19. DNA Repair (Amst). 2015. PMID: 25737057 Free PMC article. Review.
-
Polymerase dynamics at the eukaryotic DNA replication fork.J Biol Chem. 2009 Feb 13;284(7):4041-5. doi: 10.1074/jbc.R800062200. Epub 2008 Oct 3. J Biol Chem. 2009. PMID: 18835809 Free PMC article. Review.
Cited by
-
Replication of [AT/TA]25 Microsatellite Sequences by Human DNA Polymerase δ Holoenzymes Is Dependent on dNTP and RPA Levels.Biochemistry. 2024 Apr 16;63(8):969-983. doi: 10.1021/acs.biochem.4c00006. Epub 2024 Mar 26. Biochemistry. 2024. PMID: 38623046 Free PMC article.
-
Expression Profile, Molecular Association, and Clinical Significance of POLD4 in Glioblastoma.Cell Mol Neurobiol. 2023 Oct;43(7):3753-3765. doi: 10.1007/s10571-023-01393-x. Epub 2023 Aug 6. Cell Mol Neurobiol. 2023. PMID: 37543966 Free PMC article.
-
A human high-fidelity DNA polymerase holoenzyme has a wide range of lesion bypass activities.Nucleic Acids Res. 2025 Jul 8;53(13):gkaf646. doi: 10.1093/nar/gkaf646. Nucleic Acids Res. 2025. PMID: 40671520 Free PMC article.
-
PRIMPOL ensures robust handoff between on-the-fly and post-replicative DNA lesion bypass.Nucleic Acids Res. 2024 Jan 11;52(1):243-258. doi: 10.1093/nar/gkad1054. Nucleic Acids Res. 2024. PMID: 37971291 Free PMC article.
-
A human high-fidelity DNA polymerase holoenzyme has a wide range of lesion bypass activities.bioRxiv [Preprint]. 2024 Oct 17:2024.10.14.618244. doi: 10.1101/2024.10.14.618244. bioRxiv. 2024. Update in: Nucleic Acids Res. 2025 Jul 8;53(13):gkaf646. doi: 10.1093/nar/gkaf646. PMID: 39464047 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

