Single domain antibodies against enteric pathogen virulence factors are active as curli fiber fusions on probiotic E. coli Nissle 1917
- PMID: 36107831
- PMCID: PMC9477280
- DOI: 10.1371/journal.ppat.1010713
Single domain antibodies against enteric pathogen virulence factors are active as curli fiber fusions on probiotic E. coli Nissle 1917
Abstract
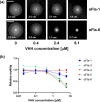
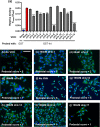
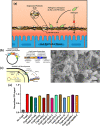
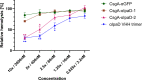
Enteric microbial pathogens, including Escherichia coli, Shigella and Cryptosporidium species, take a particularly heavy toll in low-income countries and are highly associated with infant mortality. We describe here a means to display anti-infective agents on the surface of a probiotic bacterium. Because of their stability and versatility, VHHs, the variable domains of camelid heavy-chain-only antibodies, have potential as components of novel agents to treat or prevent enteric infectious disease. We isolated and characterized VHHs targeting several enteropathogenic E. coli (EPEC) virulence factors: flagellin (Fla), which is required for bacterial motility and promotes colonization; both intimin and the translocated intimin receptor (Tir), which together play key roles in attachment to enterocytes; and E. coli secreted protein A (EspA), an essential component of the type III secretion system (T3SS) that is required for virulence. Several VHHs that recognize Fla, intimin, or Tir blocked function in vitro. The probiotic strain E. coli Nissle 1917 (EcN) produces on the bacterial surface curli fibers, which are the major proteinaceous component of E. coli biofilms. A subset of Fla-, intimin-, or Tir-binding VHHs, as well as VHHs that recognize either a T3SS of another important bacterial pathogen (Shigella flexneri), a soluble bacterial toxin (Shiga toxin or Clostridioides difficile toxin TcdA), or a major surface antigen of an important eukaryotic pathogen (Cryptosporidium parvum) were fused to CsgA, the major curli fiber subunit. Scanning electron micrographs indicated CsgA-VHH fusions were assembled into curli fibers on the EcN surface, and Congo Red binding indicated that these recombinant curli fibers were produced at high levels. Ectopic production of these VHHs conferred on EcN the cognate binding activity and, in the case of anti-Shiga toxin, was neutralizing. Taken together, these results demonstrate the potential of the curli-based pathogen sequestration strategy described herein and contribute to the development of novel VHH-based gut therapeutics.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Kotloff KL, Blackwelder WC, Nasrin D, Nataro JP, Farag TH, van Eijk A, et al.. The Global Enteric Multicenter Study (GEMS) of Diarrheal Disease in Infants and Young Children in Developing Countries: Epidemiologic and Clinical Methods of the Case/Control Study. Clinical Infectious Diseases. 2012;55(suppl_4):S232–S45. doi: 10.1093/cid/cis753 - DOI - PMC - PubMed
-
- Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, et al.. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. The Lancet. 2013;382(9888):209–22. doi: 10.1016/S0140-6736(13)60844-2 - DOI - PubMed
-
- Nicolini G, Sperotto F, Esposito S. Combating the rise of antibiotic resistance in children. Minerva Pediatr. 2014;66(1):31–9. - PubMed
-
- Kotloff KL, Nasrin D, Blackwelder WC, Wu Y, Farag T, Panchalingham S, et al.. The incidence, aetiology, and adverse clinical consequences of less severe diarrhoeal episodes among infants and children residing in low-income and middle-income countries: a 12-month case-control study as a follow-on to the Global Enteric Multicenter Study (GEMS). The Lancet Global Health. 2019;7(5):e568–e84. doi: 10.1016/S2214-109X(19)30076-2 - DOI - PMC - PubMed
-
- Levine MM, Nasrin D, Acácio S, Bassat Q, Powell H, Tennant SM, et al.. Diarrhoeal disease and subsequent risk of death in infants and children residing in low-income and middle-income countries: analysis of the GEMS case-control study and 12-month GEMS-1A follow-on study. The Lancet Global Health. 2020;8(2):e204–e14. doi: 10.1016/S2214-109X(19)30541-8 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

