UDP-glucose dehydrogenase (UGDH) activity is suppressed by peroxide and promoted by PDGF in fibroblast-like synoviocytes: Evidence of a redox control mechanism
- PMID: 36107941
- PMCID: PMC9477357
- DOI: 10.1371/journal.pone.0274420
UDP-glucose dehydrogenase (UGDH) activity is suppressed by peroxide and promoted by PDGF in fibroblast-like synoviocytes: Evidence of a redox control mechanism
Abstract
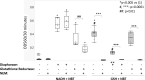
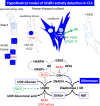
UDP-glucose dehydrogenase (UGDH) generates essential precursors of hyaluronic acid (HA) synthesis, however mechanisms regulating its activity are unclear. We used enzyme histostaining and quantitative image analysis to test whether cytokines that stimulate HA synthesis upregulate UGDH activity. Fibroblast-like synoviocytes (FLS, from N = 6 human donors with knee pain) were cultured, freeze-thawed, and incubated for 1 hour with UDP-glucose, NAD+ and nitroblue tetrazolium (NBT) which allows UGDH to generate NADH, and NADH to reduce NBT to a blue stain. Compared to serum-free medium, FLS treated with PDGF showed 3-fold higher UGDH activity and 6-fold higher HA release, but IL-1beta/TGF-beta1 induced 27-fold higher HA release without enhancing UGDH activity. In selected proliferating cells, UGDH activity was lost in the cytosol, but preserved in the nucleus. Cell-free assays led us to discover that diaphorase, a cytosolic enzyme, or glutathione reductase, a nuclear enzyme, was necessary and sufficient for NADH to reduce NBT to a blue formazan dye in a 1-hour timeframe. Primary synovial fibroblasts and transformed A549 fibroblasts showed constitutive diaphorase/GR staining activity that varied according to supplied NADH levels, with relatively stronger UGDH and diaphorase activity in A549 cells. Unilateral knee injury in New Zealand White rabbits (N = 3) stimulated a coordinated increase in synovial membrane UGDH and diaphorase activity, but higher synovial fluid HA in only 2 out of 3 injured joints. UGDH activity (but not diaphorase) was abolished by N-ethyl maleimide, and inhibited by peroxide or UDP-xylose. Our results do not support the hypothesis that UGDH is a rate-liming enzyme for HA synthesis under catabolic inflammatory conditions that can oxidize and inactivate the UGDH active site cysteine. Our novel data suggest a model where UGDH activity is controlled by a redox switch, where intracellular peroxide inactivates, and high glutathione and diaphorase promote UGDH activity by maintaining the active site cysteine in a reduced state, and by recycling NAD+ from NADH.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: CDH, Scientific Advisory Board and a shareholder of Chitogenx Inc. (formerly Ortho RTi Inc.); RC, CM, RS, AC, DF, RM, HEG, SA and MP have declared that no competing interests exist. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures





References
-
- Egger S, Chaikuad A, Klimacek M, Kavanagh KL, Oppermann U, Nidetzky B. Structural and kinetic evidence that catalytic reaction of human UDP-glucose 6-dehydrogenase involves covalent thiohemiacetal and thioester enzyme intermediates. J Biol Chem. 2011/11/28 ed. 2012;287: 2119–2129. doi: 10.1074/jbc.M111.313015 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

