Neuroprotective Effects of σ2R/TMEM97 Receptor Modulators in the Neuronal Model of Huntington's Disease
- PMID: 36108101
- PMCID: PMC9547941
- DOI: 10.1021/acschemneuro.2c00274
Neuroprotective Effects of σ2R/TMEM97 Receptor Modulators in the Neuronal Model of Huntington's Disease
Abstract
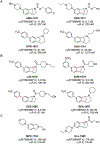
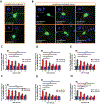
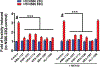
Huntington's disease (HD) is a genetic neurodegenerative disease caused by an expanded CAG repeat in the Huntingtin (HTT) gene that encodes for an expanded polyglutamine (polyQ) repeat in exon-1 of the human mutant huntingtin (mHTT) protein. The presence of this polyQ repeat results in neuronal degeneration, for which there is no cure or treatment that modifies disease progression. In previous studies, we have shown that small molecules that bind selectively to σ2R/TMEM97 can have significant neuroprotective effects in models of Alzheimer's disease, traumatic brain injury, and several other neurodegenerative diseases. In the present work, we extend these investigations and show that certain σ2R/TMEM97-selective ligands decrease mHTT-induced neuronal toxicity. We first synthesized a set of compounds designed to bind to σ2R/TMEM97 and determined their binding profiles (Ki values) for σ2R/TMEM97 and other proteins in the central nervous system. Modulators with high affinity and selectivity for σ2R/TMEM97 were then tested in our HD cell model. Primary cortical neurons were cultured in vitro for 7 days and then co-transfected with either a normal HTT construct (Htt N-586-22Q/GFP) or the mHTT construct Htt-N586-82Q/GFP. Transfected neurons were treated with either σ2R/TMEM97 or σ1R modulators for 48 h. After treatment, neurons were fixed and stained with Hoechst, and condensed nuclei were quantified to assess cell death in the transfected neurons. Significantly, σ2R/TMEM97 modulators reduce the neuronal toxicity induced by mHTT, and their neuroprotective effects are not blocked by NE-100, a selective σ1R antagonist known to block neuroprotection by σ1R ligands. These results indicate for the first time that σ2R/TMEM97 modulators can protect neurons from mHTT-induced neuronal toxicity, suggesting that targeting σ2R/TMEM97 may lead to a novel therapeutic approach to treat patients with HD.
Keywords: Huntington’s disease; neuronal survival; neuroprotection; nucleus condensation; σ2R/TMEM97.
Conflict of interest statement
Figures
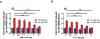
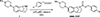


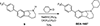

References
-
- Tabrizi SJ, Flower MD, Ross CA, and Wild EJ (2020) Huntington disease: new insights into molecular pathogenesis and therapeutic opportunities, Nat Rev Neurol 16, 529–546. - PubMed
-
- (1993) A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. The Huntington’s Disease Collaborative Research Group, Cell 72, 971–983. - PubMed
-
- Walker FO (2007) Huntington’s Disease, Semin Neurol 27, 143–150. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

