The Essential Role of the Choriocapillaris in Vision: Novel Insights from Imaging and Molecular Biology
- PMID: 36108103
- PMCID: PMC9668353
- DOI: 10.1146/annurev-vision-100820-085958
The Essential Role of the Choriocapillaris in Vision: Novel Insights from Imaging and Molecular Biology
Abstract
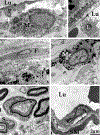
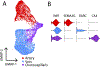
The choriocapillaris, a dense capillary network located at the posterior pole of the eye, is essential for supporting normal vision, supplying nutrients, and removing waste products from photoreceptor cells and the retinal pigment epithelium. The anatomical location, heterogeneity, and homeostatic interactions with surrounding cell types make the choroid complex to study both in vivo and in vitro. Recent advances in single-cell RNA sequencing, in vivo imaging, and in vitro cell modeling are vastly improving our knowledge of the choroid and its role in normal health and in age-related macular degeneration (AMD). Histologically, loss of endothelial cells (ECs) of the choriocapillaris occurs early in AMD concomitant with elevated formation of the membrane attack complex of complement. Advanced imaging has allowed us to visualize early choroidal blood flow changes in AMD in living patients, supporting histological findings of loss of choroidal ECs. Single-cell RNA sequencing is being used to characterize choroidal cell types transcriptionally and discover their altered patterns of gene expression in aging and disease. Advances in induced pluripotent stem cell protocols and 3D cultures will allow us to closely mimic the in vivo microenvironment of the choroid in vitro to better understand the mechanism leading to choriocapillaris loss in AMD.
Keywords: age-related macular degeneration; choroid; endothelial cell; iPSC; induced pluripotent stem cell; single-cell RNA sequencing.
Figures






Similar articles
-
Structural and molecular changes in the aging choroid: implications for age-related macular degeneration.Eye (Lond). 2017 Jan;31(1):10-25. doi: 10.1038/eye.2016.216. Epub 2016 Oct 7. Eye (Lond). 2017. PMID: 27716746 Free PMC article. Review.
-
Increased cell stiffness contributes to complement-mediated injury of choroidal endothelial cells in a monkey model of early age-related macular degeneration.J Pathol. 2022 Jul;257(3):314-326. doi: 10.1002/path.5892. Epub 2022 Apr 8. J Pathol. 2022. PMID: 35239183 Free PMC article.
-
Single-cell transcriptomics of the human retinal pigment epithelium and choroid in health and macular degeneration.Proc Natl Acad Sci U S A. 2019 Nov 26;116(48):24100-24107. doi: 10.1073/pnas.1914143116. Epub 2019 Nov 11. Proc Natl Acad Sci U S A. 2019. PMID: 31712411 Free PMC article.
-
The membrane attack complex in aging human choriocapillaris: relationship to macular degeneration and choroidal thinning.Am J Pathol. 2014 Nov;184(11):3142-53. doi: 10.1016/j.ajpath.2014.07.017. Epub 2014 Sep 7. Am J Pathol. 2014. PMID: 25204844 Free PMC article.
-
Complement activation and choriocapillaris loss in early AMD: implications for pathophysiology and therapy.Prog Retin Eye Res. 2015 Mar;45:1-29. doi: 10.1016/j.preteyeres.2014.11.005. Epub 2014 Dec 5. Prog Retin Eye Res. 2015. PMID: 25486088 Free PMC article. Review.
Cited by
-
A Novel Grid Strategy for Correlating Focal Macular Anatomic Changes With Focal Changes in Choriocapillaris Perfusion.Invest Ophthalmol Vis Sci. 2024 Dec 2;65(14):5. doi: 10.1167/iovs.65.14.5. Invest Ophthalmol Vis Sci. 2024. PMID: 39625442 Free PMC article.
-
Key role for inflammation-related signaling in the pathogenesis of myopia based on evidence from proteomics analysis.Sci Rep. 2024 Oct 8;14(1):23486. doi: 10.1038/s41598-024-67337-7. Sci Rep. 2024. PMID: 39379387 Free PMC article.
-
Importance of Choriocapillaris Replacement in Therapeutic Strategies for Age-Related Macular Degeneration.Adv Exp Med Biol. 2025;1468:389-393. doi: 10.1007/978-3-031-76550-6_64. Adv Exp Med Biol. 2025. PMID: 39930227 Review.
-
The Impact of Carotid Endarterectomy on Choriocapillaris Perfusion.Invest Ophthalmol Vis Sci. 2023 Dec 1;64(15):42. doi: 10.1167/iovs.64.15.42. Invest Ophthalmol Vis Sci. 2023. PMID: 38153750 Free PMC article.
-
High-Resolution Optical Coherence Tomography in Healthy Individuals Provides Resolution at the Cellular and Subcellular Levels.Transl Vis Sci Technol. 2023 Jul 3;12(7):12. doi: 10.1167/tvst.12.7.12. Transl Vis Sci Technol. 2023. PMID: 37428129 Free PMC article.
References
-
- Macular Photocoagulation Study Group 1991. Subfoveal neovascular lesions in age-related macular degeneration. Guidelines for evaluation and treatment in the macular photocoagulation study. Archives of ophthalmology (Chicago, Ill. : 1960) 109, 1242–1257. - PubMed
-
- Ames A 3rd, 1992. Energy requirements of CNS cells as related to their function and to their vulnerability to ischemia: a commentary based on studies on retina. Can J Physiol Pharmacol 70 Suppl, S158–164. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

