Outcomes After Nonresponse and Relapse Post-Tisagenlecleucel in Children, Adolescents, and Young Adults With B-Cell Acute Lymphoblastic Leukemia
- PMID: 36108252
- PMCID: PMC9839307
- DOI: 10.1200/JCO.22.01076
Outcomes After Nonresponse and Relapse Post-Tisagenlecleucel in Children, Adolescents, and Young Adults With B-Cell Acute Lymphoblastic Leukemia
Abstract
Purpose: Nonresponse and relapse after CD19-chimeric antigen receptor (CAR) T-cell therapy continue to challenge survival outcomes. Phase II landmark data from the ELIANA trial demonstrated nonresponse and relapse rates of 14.5% and 28%, respectively, whereas use in the real-world setting showed nonresponse and relapse rates of 15% and 37%. Outcome analyses describing fate after post-CAR nonresponse and relapse remain limited. Here, we aim to establish survival outcomes after nonresponse and both CD19+ and CD19- relapses and explore treatment variables associated with inferior survival.
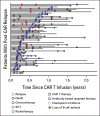
Methods: We conducted a retrospective multi-institutional study of 80 children and young adults with B-cell acute lymphoblastic leukemia experiencing nonresponse (n = 23) or relapse (n = 57) after tisagenlecleucel. We analyze associations between baseline characteristics and these outcomes and establish survival rates and salvage approaches.
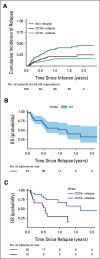
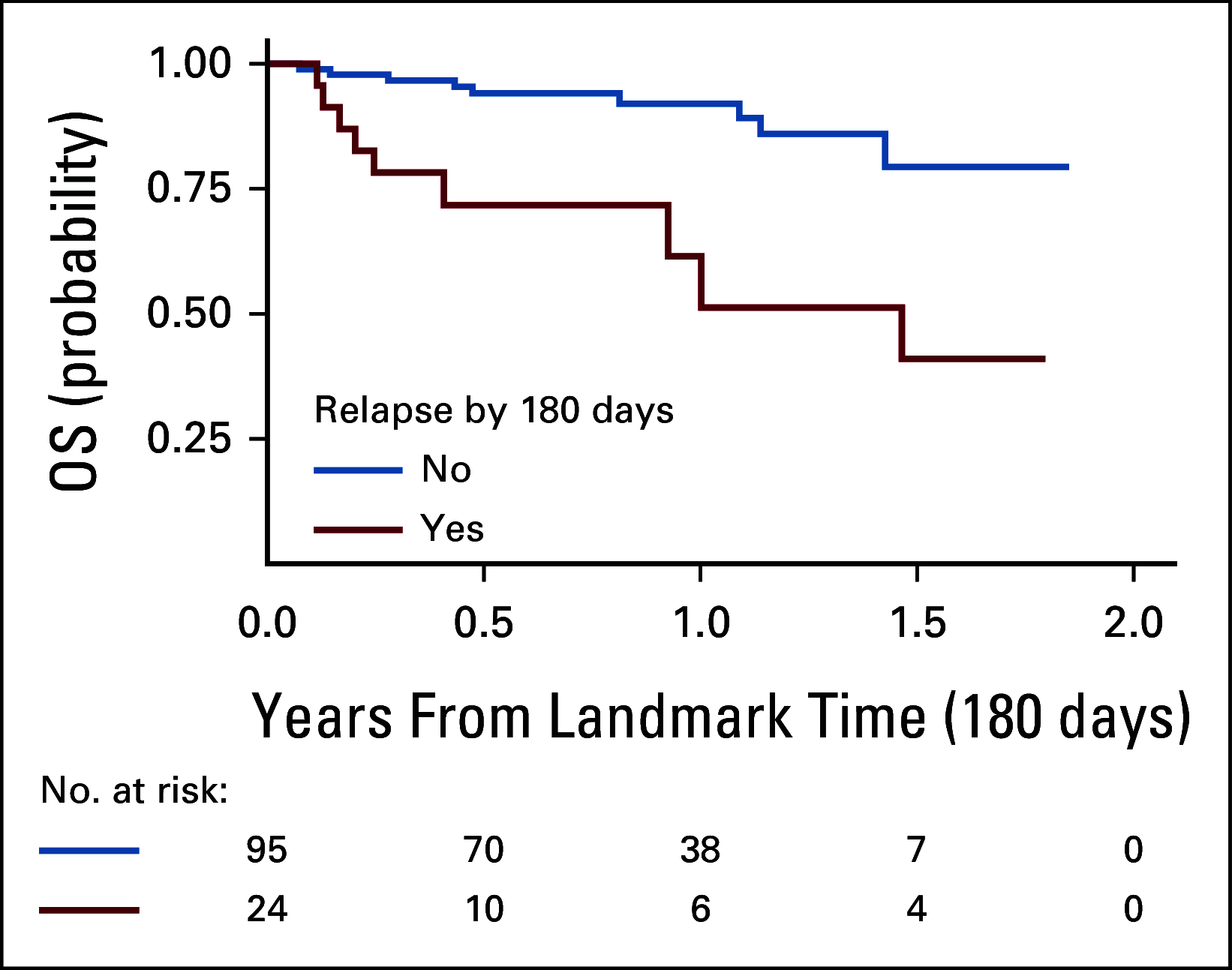
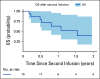
Results: The overall survival (OS) at 12 months was 19% across nonresponders (n = 23; 95% CI, 7 to 50). Ninety-five percent of patients with nonresponse had high preinfusion disease burden. Among 156 morphologic responders, the cumulative incidence of relapse was 37% (95% CI, 30 to 47) at 12 months (CD19+; 21% [15 to 29], CD19-; 16% [11 to 24], median follow-up; 380 days). Across 57 patients experiencing relapse, the OS was 52% (95% CI, 38 to 71) at 12 months after time of relapse. Notably, CD19- relapse was associated with significantly decreased OS as compared with patients who relapsed with conserved CD19 expression (CD19- 12-month OS; 30% [14 to 66], CD19+ 12-month OS; 68% [49 to 92], P = .0068). Inotuzumab, CAR reinfusion, and chemotherapy were used as postrelapse salvage therapy with greatest frequency, yet high variability in treatment sequencing and responses limits efficacy analysis across salvage approaches.
Conclusion: We describe poor survival across patients experiencing nonresponse to tisagenlecleucel. In the post-tisagenlecleucel relapse setting, patients can be salvaged; however, CD19- relapse is distinctly associated with decreased survival outcomes.
Conflict of interest statement
No other potential conflicts of interest were reported.
Figures


Comment in
-
Challenges in the treatment of pediatric acute lymphoblastic leukemia: insights from the pediatric real world CAR consortium regarding nonresponse and relapse post tisagenlecleucel.Transl Pediatr. 2023 Dec 26;12(12):2095-2098. doi: 10.21037/tp-23-421. Epub 2023 Dec 19. Transl Pediatr. 2023. PMID: 38197106 Free PMC article. No abstract available.

