Genomic Classification of HER2-Positive Patients With 80-Gene and 70-Gene Signatures Identifies Diversity in Clinical Outcomes With HER2-Targeted Neoadjuvant Therapy
- PMID: 36108259
- PMCID: PMC9489196
- DOI: 10.1200/PO.22.00197
Genomic Classification of HER2-Positive Patients With 80-Gene and 70-Gene Signatures Identifies Diversity in Clinical Outcomes With HER2-Targeted Neoadjuvant Therapy
Abstract
Purpose: The prospective Neoadjuvant Breast Registry Symphony Trial compared the 80-gene molecular subtyping signature with clinical assessment by immunohistochemistry and/or fluorescence in situ hybridization in predicting pathologic complete response (pCR) and 5-year outcomes in patients with early-stage breast cancer.
Methods: Standard-of-care neoadjuvant chemotherapy combined with trastuzumab or trastuzumab plus pertuzumab was given to patients with human epidermal growth factor receptor 2 (HER2)-positive tumors (n = 295). pCR was the primary end point, with secondary end points of distant metastasis-free survival and overall survival at 5 years.
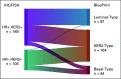
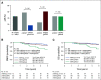
Results: Among clinically defined HER2-positive (cHER2) tumors, the 80-gene assay identified 29.5% (87 of 295) as Luminal-Type (cHER2/gLuminal), 14.9% (44 of 295) as Basal-Type (cHER2/gBasal), and 55.6% (164 of 295) as HER2-Type (cHER2/genomically classified as HER2 [gHER2]). Patients with cHER2/gHER2 tumors had a higher pCR rate (61.6%) compared with non-gHER2 tumors (26.7%; P < .001). Dual targeting for cHER2/gHER2 tumors yielded a higher pCR rate (75%) compared with those treated with single HER2-targeted therapy (54%; P = .006). For cHER2/gBasal tumors, the 42.9% pCR rate observed with dual targeting was not different from that with trastuzumab alone (46.4%; P = .830). Among those with cHER2/gBasal tumors, 5-year distant metastasis-free survival (68.6%; 95% CI, 49.1 to 81.9) was significantly worse than in patients with cHER2/gLuminal tumors (88.9%; 95% CI, 78.0 to 94.6) and cHER2/gHER2 tumors (87.4%; 95% CI, 80.2 to 92.2; P = .010), with similar corresponding overall survival differences.
Conclusion: The 80-gene assay identified meaningful genomic diversity in patients with cHER2 disease. Patients with cHER2/gHER2 tumors, who benefitted most from dual HER2-targeted therapy, accounted for approximately half of the cHER2 cohort. Genomically Luminal tumors had low pCR rates but good 5-year outcomes. cHER2/gBasal tumors derived no benefit from dual therapy and had significantly worse 5-year prognosis; these patients merit special consideration in future trials.
Trial registration: ClinicalTrials.gov NCT01479101.
Conflict of interest statement
<b>Pat W. Whitworth</b><b>Employment:</b> Integra LifeSciences (I)<b>Leadership:</b> Integra LifeSciences (I)<b>Stock and Other Ownership Interests:</b> Targeted Medical Education Inc, Integra LifeSciences (I)<b>Honoraria:</b> Puma Biotechnology<b>Consulting or Advisory Role:</b> ImpediMed, Prelude Therapeutics, Becton Dickinson<b>Research Funding:</b> Prelude Therapeutics, Agendia, Medneon<b>Travel, Accommodations, Expenses:</b> Targeted Medical Education Inc <b>Peter D. Beitsch</b><b>Employment:</b> Invitae<b>Leadership:</b> Targeted Medical Education Inc<b>Stock and Other Ownership Interests:</b> Targeted Medical Education Inc, Invitae<b>Research Funding:</b> Invitae<b>Expert Testimony:</b> Dune Medical Devices, ImpediMed<b>Uncompensated Relationships:</b> Medneon <b>Paul D. Richards</b><b>Stock and Other Ownership Interests:</b> NanoViricides<b>Research Funding:</b> Carrick Therapeutics (Inst) <b>James V. Pellicane</b><b>Stock and Other Ownership Interests:</b> PreludeDx<b>Honoraria:</b> Agendia, PreludeDx<b>Speakers' Bureau:</b> Agendia, PreludeDx <b>Beth B. Dupree</b><b>Leadership:</b> Caliber Medical<b>Stock and Other Ownership Interests:</b> Videra Surgical<b>Honoraria:</b> Medtronic, Perimeter Medical <b>William Dooley</b><b>Leadership:</b> Shaga Medical LLC<b>Stock and Other Ownership Interests:</b> Shaga Medical<b>Research Funding:</b> Agendia, Xoft<b>Patents, Royalties, Other Intellectual Property:</b> patent pending—microendoscopy system <b>Shiyu Wang</b><b>Employment:</b> Agendia <b>Patricia Dauer</b><b>Employment:</b> Agendia<b>Stock and Other Ownership Interests:</b> Agendia<b>Travel, Accommodations, Expenses:</b> Agendia <b>Andrea R. Menicucci</b><b>Employment:</b> Agendia <b>Erin B. Yoder</b><b>Employment:</b> Agendia<b>Stock and Other Ownership Interests:</b> Agendia<b>Travel, Accommodations, Expenses:</b> Agendia <b>Lisa E. Blumencranz</b><b>Employment:</b> Agendia <b>William Audeh</b><b>Employment:</b> Agendia<b>Leadership:</b> Agendia<b>Stock and Other Ownership Interests:</b> Agendia<b>Consulting or Advisory Role:</b> Celanese, Private Health<b>Research Funding:</b> Agendia<b>Travel, Accommodations, Expenses:</b> AgendiaNo other potential conflicts of interest were reported.
Figures


References
-
- Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. - PubMed
-
- Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): A randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:25–32. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

