Recombinant Erwinia asparaginase (JZP458) in acute lymphoblastic leukemia: results from the phase 2/3 AALL1931 study
- PMID: 36108304
- PMCID: PMC10651770
- DOI: 10.1182/blood.2022016923
Recombinant Erwinia asparaginase (JZP458) in acute lymphoblastic leukemia: results from the phase 2/3 AALL1931 study
Abstract
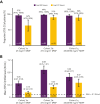
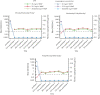
AALL1931, a phase 2/3 study conducted in collaboration with the Children's Oncology Group, investigated the efficacy and safety of JZP458 (asparaginase erwinia chrysanthemi [recombinant]-rywn), a recombinant Erwinia asparaginase derived from a novel expression platform, in patients with acute lymphoblastic leukemia/lymphoblastic lymphoma who developed hypersensitivity/silent inactivation to Escherichia coli-derived asparaginases. Each dose of a pegylated E coli-derived asparaginase remaining in patients' treatment plan was substituted by 6 doses of intramuscular (IM) JZP458 on Monday/Wednesday/Friday (MWF). Three regimens were evaluated: cohort 1a, 25 mg/m2 MWF; cohort 1b, 37.5 mg/m2 MWF; and cohort 1c, 25/25/50 mg/m2 MWF. Efficacy was evaluated by the proportion of patients maintaining adequate nadir serum asparaginase activity (NSAA ≥0.1 IU/mL) at 72 hours and at 48 hours during the first treatment course. A total of 167 patients were enrolled: cohort 1a (n = 33), cohort 1b (n = 83), and cohort 1c (n = 51). Mean serum asparaginase activity levels (IU/mL) at 72 hours were cohort 1a, 0.16, cohort 1b, 0.33, and cohort 1c, 0.47, and at 48 hours were 0.45, 0.88, and 0.66, respectively. The proportion of patients achieving NSAA ≥0.1 IU/mL at 72 and 48 hours in cohort 1c was 90% (44/49) and 96% (47/49), respectively. Simulated data from a population pharmacokinetic model matched the observed data well. Grade 3/4 treatment-related adverse events occurred in 86 of 167 (51%) patients; those leading to discontinuation included pancreatitis (6%), allergic reactions (5%), increased alanine aminotransferase (1%), and hyperammonemia (1%). Results demonstrate that IM JZP458 at 25/25/50 mg/m2 MWF is efficacious and has a safety profile consistent with other asparaginases. This trial was registered at www.clinicaltrials.gov as #NCT04145531.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: L.M. served on an advisory board and speakers bureau for Jazz Pharmaceuticals. M.R.C., T.L., E.A., M.Z., S.A., and R.I. are employees of and hold stock ownership and/or stock options in Jazz Pharmaceuticals. J.A.S. was an employee of Jazz Pharmaceuticals at the time of the study and holds stock ownership and/or stock options in Jazz Pharmaceuticals. L.B.S. served on scientific advisory boards for Jazz Pharmaceuticals and Servier Pharmaceuticals. E.A.R. received institutional research funding from Pfizer and serves on a Data and Safety Monitoring Board for Bristol Myers Squibb. R.E.R. served on advisory boards for Jazz Pharmaceuticals and Servier Pharmaceuticals. The remaining authors declare no competing financial interests.
Michelle Zanette died on 30 June 2022.
Figures


Comment in
-
JZP458 closes the asparaginase allergy gap.Blood. 2023 Feb 16;141(7):685-686. doi: 10.1182/blood.2022018395. Blood. 2023. PMID: 36795447 No abstract available.
References
-
- Raetz EA, Salzer WL. Tolerability and efficacy of L-asparaginase therapy in pediatric patients with acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2010;32(7):554–563. - PubMed
-
- Willer A, Gerss J, König T, et al. Anti-Escherichia coli asparaginase antibody levels determine the activity of second-line treatment with pegylated E coli asparaginase: a retrospective analysis within the ALL-BFM trials. Blood. 2011;118(22):5774–5782. - PubMed

