Structures of α-synuclein filaments from human brains with Lewy pathology
- PMID: 36108674
- PMCID: PMC7613749
- DOI: 10.1038/s41586-022-05319-3
Structures of α-synuclein filaments from human brains with Lewy pathology
Abstract
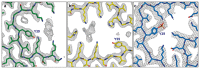
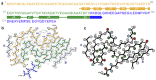
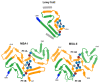
Parkinson's disease (PD) is the most common movement disorder, with resting tremor, rigidity, bradykinesia and postural instability being major symptoms1. Neuropathologically, it is characterized by the presence of abundant filamentous inclusions of α-synuclein in the form of Lewy bodies and Lewy neurites in some brain cells, including dopaminergic nerve cells of the substantia nigra2. PD is increasingly recognised as a multisystem disorder, with cognitive decline being one of its most common non-motor symptoms. Many patients with PD develop dementia more than 10 years after diagnosis3. PD dementia (PDD) is clinically and neuropathologically similar to dementia with Lewy bodies (DLB), which is diagnosed when cognitive impairment precedes parkinsonian motor signs or begins within one year from their onset4. In PDD, cognitive impairment develops in the setting of well-established PD. Besides PD and DLB, multiple system atrophy (MSA) is the third major synucleinopathy5. It is characterized by the presence of abundant filamentous α-synuclein inclusions in brain cells, especially oligodendrocytes (Papp-Lantos bodies). We previously reported the electron cryo-microscopy structures of two types of α-synuclein filament extracted from the brains of individuals with MSA6. Each filament type is made of two different protofilaments. Here we report that the cryo-electron microscopy structures of α-synuclein filaments from the brains of individuals with PD, PDD and DLB are made of a single protofilament (Lewy fold) that is markedly different from the protofilaments of MSA. These findings establish the existence of distinct molecular conformers of assembled α-synuclein in neurodegenerative disease.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures







Comment in
-
Identification of Novel α-Synuclein Assemblies in Lewy Body Disease.Mov Disord. 2023 Jan;38(1):21-22. doi: 10.1002/mds.29272. Epub 2022 Nov 14. Mov Disord. 2023. PMID: 36374937 Free PMC article. No abstract available.
References
-
- Goedert M, Spillantini MG, Del Tredici K, Braak H. 100 years of Lewy pathology. Nature Rev Neurol. 2013;9:13–24. - PubMed
-
- Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh-Sørensen P. Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Arch Neurol. 2003;60:387–392. - PubMed
-
- Fanciulli A, Wenning GK. Multiple system atrophy. N Engl J Med. 2015;372:249–263. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

