Targeting FoxO transcription factors with HDAC inhibitors for the treatment of osteoarthritis
- PMID: 36109140
- PMCID: PMC11005918
- DOI: 10.1136/ard-2021-221269
Targeting FoxO transcription factors with HDAC inhibitors for the treatment of osteoarthritis
Abstract
Objectives: Osteoarthritis (OA) features ageing-related defects in cellular homeostasis mechanisms in articular cartilage. These defects are associated with suppression of forkhead box O (FoxO) transcription factors. FoxO1 or FoxO3 deficient mice show early onset OA while FoxO1 protects against oxidative stress in chondrocytes and promotes expression of autophagy genes and the essential joint lubricant proteoglycan 4 (PRG4). The objective of this study was to identify small molecules that can increase FoxO1 expression.
Methods: We constructed a reporter cell line with FoxO1 promoter sequences and performed high-throughput screening (HTS) of the Repurposing, Focused Rescue and Accelerated Medchem (ReFRAME) library . Hits from the HTS were validated and function was assessed in human chondrocytes, meniscus cells and synoviocytes and following administration to mice. The most promising hit, the histone deacetylase inhibitor (HDACI) panobinostat was tested in a murine OA model.
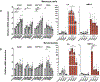
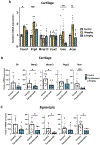
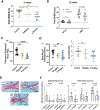
Results: Among the top hits were HDACI and testing in human chondrocytes, meniscus cells and synoviocytes showed that panobinostat was the most promising compound as it increased the expression of autophagy genes and PRG4 while suppressing the basal and IL-1β induced expression of inflammatory mediators and extracellular matrix degrading enzymes. Intraperitoneal administration of panobinostat also suppressed the expression of mediators of OA pathogenesis induced by intra-articular injection of IL-1β. In a murine OA model, panobinostat reduced the severity of histological changes in cartilage, synovium and subchondral bone and improved pain behaviours.
Conclusion: Panobinostat has a clinically relevant activity profile and is a candidate for OA symptom and structure modification.
Keywords: Chondrocytes; Inflammation; Osteoarthritis.
© Author(s) (or their employer(s)) 2023. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures


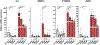
References
-
- Courties A, Berenbaum F, Sellam J. The Phenotypic Approach to Osteoarthritis: A Look at Metabolic Syndrome-Associated Osteoarthritis. Joint Bone Spine 2019;86:725–30. - PubMed
-
- Hawker GA. Osteoarthritis is a serious disease. Clin Exp Rheumatol 2019;37 Suppl 120:3–6. - PubMed
-
- Biver E, Berenbaum F, Valdes AM, Araujo de Carvalho I, Bindels LB, Brandi ML, et al. Gut microbiota and osteoarthritis management: An expert consensus of the European society for clinical and economic aspects of osteoporosis, osteoarthritis and musculoskeletal diseases (ESCEO). Ageing Res Rev 2019;55:100946. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

