Beauty of the beast: anticholinergic tropane alkaloids in therapeutics
- PMID: 36109439
- PMCID: PMC9478010
- DOI: 10.1007/s13659-022-00357-w
Beauty of the beast: anticholinergic tropane alkaloids in therapeutics
Abstract
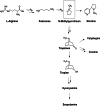
Tropane alkaloids (TAs) are among the most valued chemical compounds known since pre-historic times. Poisonous plants from Solanaceae family (Hyoscyamus niger, Datura, Atropa belladonna, Scopolia lurida, Mandragora officinarum, Duboisia) and Erythroxylaceae (Erythroxylum coca) are rich sources of tropane alkaloids. These compounds possess the anticholinergic properties as they could block the neurotransmitter acetylcholine action in the central and peripheral nervous system by binding at either muscarinic and/or nicotinic receptors. Hence, they are of great clinical importance and are used as antiemetics, anesthetics, antispasmodics, bronchodilator and mydriatics. They also serve as the lead compounds to generate more effective drugs. Due to the important pharmacological action they are listed in the WHO list of essential medicines and are available in market with FDA approval. However, being anticholinergic in action, TA medication are under the suspicion of causing dementia and cognitive decline like other medications with anticholinergic action, interestingly which is incorrect. There are published reviews on chemistry, biosynthesis, pharmacology, safety concerns, biotechnological aspects of TAs but the detailed information on anticholinergic mechanism of action, clinical pharmacology, FDA approval and anticholinergic burden is lacking. Hence the present review tries to fill this lacuna by critically summarizing and discussing the above mentioned aspects.
Keywords: Anticholinergic action; Anticholinergic burden; Muscarinic and nicotinic receptors; Poisonous plants; Therapeutics; Tropane alkaloids.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Simultaneous quantification of hyoscyamine and scopolamine using HPLC-DAD in four Solanaceae: Hyoscyamus niger, Datura stramonium, Atropa belladonna and Mandragora officinarum.Nat Prod Res. 2025 Feb;39(3):438-443. doi: 10.1080/14786419.2023.2269595. Epub 2023 Oct 13. Nat Prod Res. 2025. PMID: 37830795
-
Common anticholinergic solanaceaous plants of temperate Europe - A review of intoxications from the literature (1966-2018).Toxicon. 2020 Apr 15;177:52-88. doi: 10.1016/j.toxicon.2020.02.005. Epub 2020 Feb 13. Toxicon. 2020. PMID: 32217234 Review.
-
Elucidation of tropane alkaloid biosynthesis in Erythroxylum coca using a microbial pathway discovery platform.Proc Natl Acad Sci U S A. 2022 Dec 6;119(49):e2215372119. doi: 10.1073/pnas.2215372119. Epub 2022 Nov 28. Proc Natl Acad Sci U S A. 2022. PMID: 36442128 Free PMC article.
-
Tropane Alkaloids: Chemistry, Pharmacology, Biosynthesis and Production.Molecules. 2019 Feb 22;24(4):796. doi: 10.3390/molecules24040796. Molecules. 2019. PMID: 30813289 Free PMC article. Review.
-
Tropane alkaloids as medicinally useful natural products and their synthetic derivatives as new drugs.Pharmacol Rep. 2008 Jul-Aug;60(4):439-63. Pharmacol Rep. 2008. PMID: 18799813 Review.
Cited by
-
Exogenous regulation of macronutrients promotes the accumulation of alkaloid yield in anisodus tanguticus (Maxim.) pascher.BMC Plant Biol. 2024 Jun 26;24(1):602. doi: 10.1186/s12870-024-05299-8. BMC Plant Biol. 2024. PMID: 38926662 Free PMC article.
-
Natural Compounds for Preventing Age-Related Diseases and Cancers.Int J Mol Sci. 2024 Jul 9;25(14):7530. doi: 10.3390/ijms25147530. Int J Mol Sci. 2024. PMID: 39062777 Free PMC article. Review.
-
Rapid Synthesis of Psychoplastogenic Tropane Alkaloids.JACS Au. 2023 Oct 5;3(10):2703-2708. doi: 10.1021/jacsau.3c00472. eCollection 2023 Oct 23. JACS Au. 2023. PMID: 37885569 Free PMC article.
-
From Plants to Wound Dressing and Transdermal Delivery of Bioactive Compounds.Plants (Basel). 2023 Jul 16;12(14):2661. doi: 10.3390/plants12142661. Plants (Basel). 2023. PMID: 37514275 Free PMC article. Review.
-
Toxic and Hallucinogenic Plants of Southern Chile of Forensic Interest: A Review.Plants (Basel). 2025 Jul 16;14(14):2196. doi: 10.3390/plants14142196. Plants (Basel). 2025. PMID: 40733433 Free PMC article. Review.
References
-
- Lounasmaa M, Tamminen T. The tropane alkaloids. Alkaloids. 1993;44:1–114.
-
- Zeng J, et al. Analyzing the contents of tropane alkaloids in Scopolia lurida, a resource plant species of Tibetan medicines. Sci Technol Tibet. 2016;279:60–62.
-
- Diaz JL. Sacred plants and visionary consciousness. Phenomenol Cogn Sci. 2010;9(2):159–170. doi: 10.1007/s11097-010-9157-z. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

