Unbiased approach to identify and assess efficacy of human SARS-CoV-2 neutralizing antibodies
- PMID: 36109550
- PMCID: PMC9476467
- DOI: 10.1038/s41598-022-19780-7
Unbiased approach to identify and assess efficacy of human SARS-CoV-2 neutralizing antibodies
Abstract
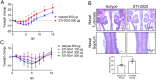
Coronavirus disease 2019 (COVID-19) continues to significantly impact the global population, thus countermeasure platforms that enable rapid development of therapeutics against variants of SARS-CoV-2 are essential. We report use of a phage display human antibody library approach to rapidly identify neutralizing antibodies (nAbs) against SARS-CoV-2. We demonstrate the binding and neutralization capability of two nAbs, STI-2020 and STI-5041, against the SARS-CoV-2 WA-1 strain as well as the Alpha and Beta variants. STI-2020 and STI-5041 were protective when administered intravenously or intranasally in the golden (Syrian) hamster model of COVID-19 challenged with the WA-1 strain or Beta variant. The ability to administer nAbs intravenously and intranasally may have important therapeutic implications and Phase 1 healthy subjects clinical trials are ongoing.
© 2022. The Author(s).
Conflict of interest statement
Sorrento authors own option and/or stock of the company. SP has financial interest in Sorrento. This work has been described in one or more provisional patent applications. HJ is an officer at Sorrento Therapeutics, Inc.. All other authors have no conflicts of interest to report.
Figures



References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

