Variability of strain engraftment and predictability of microbiome composition after fecal microbiota transplantation across different diseases
- PMID: 36109637
- PMCID: PMC9499858
- DOI: 10.1038/s41591-022-01964-3
Variability of strain engraftment and predictability of microbiome composition after fecal microbiota transplantation across different diseases
Abstract
Fecal microbiota transplantation (FMT) is highly effective against recurrent Clostridioides difficile infection and is considered a promising treatment for other microbiome-related disorders, but a comprehensive understanding of microbial engraftment dynamics is lacking, which prevents informed applications of this therapeutic approach. Here, we performed an integrated shotgun metagenomic systematic meta-analysis of new and publicly available stool microbiomes collected from 226 triads of donors, pre-FMT recipients and post-FMT recipients across eight different disease types. By leveraging improved metagenomic strain-profiling to infer strain sharing, we found that recipients with higher donor strain engraftment were more likely to experience clinical success after FMT (P = 0.017) when evaluated across studies. Considering all cohorts, increased engraftment was noted in individuals receiving FMT from multiple routes (for example, both via capsules and colonoscopy during the same treatment) as well as in antibiotic-treated recipients with infectious diseases compared with antibiotic-naïve patients with noncommunicable diseases. Bacteroidetes and Actinobacteria species (including Bifidobacteria) displayed higher engraftment than Firmicutes except for six under-characterized Firmicutes species. Cross-dataset machine learning predicted the presence or absence of species in the post-FMT recipient at 0.77 average AUROC in leave-one-dataset-out evaluation, and highlighted the relevance of microbial abundance, prevalence and taxonomy to infer post-FMT species presence. By exploring the dynamics of microbiome engraftment after FMT and their association with clinical variables, our study uncovered species-specific engraftment patterns and presented machine learning models able to predict donors that might optimize post-FMT specific microbiome characteristics for disease-targeted FMT protocols.
© 2022. The Author(s).
Conflict of interest statement
A.G. reports personal fees for consultancy for Eisai S.r.l., 3PSolutions, Real Time Meeting, Fondazione Istituto Danone, Sinergie S.r.l. Board MRGE and SanofiS.p.A; personal fees for acting as a speaker for Takeda S.p.A, AbbVie and Sandoz S.p.A; and personal fees for acting on advisory boards for VSL3 and Eisai. G.C. has received personal fees for acting as advisor for Ferring Therapeutics. G.I. has received personal fees for acting as speaker for Biocodex, Danone, Sofar, Malesci, Metagenics and Tillotts Pharma, and for acting as consultant/advisor for Ferring Therapeutics, Giuliani, Metagenics and Tillotts Pharma. N.S. reports consultancy and/or SAB contracts with Zoe, Roche, Ysopia, and Freya, Alia Therapeutics, speaker fees by Illumina, and is cofounder of PreBiomics. The other authors have no potential competing interest to disclose.
Figures




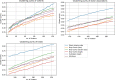
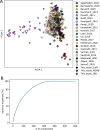
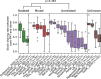




Comment in
-
Understanding and predicting the efficacy of FMT.Nat Med. 2022 Sep;28(9):1759-1760. doi: 10.1038/s41591-022-01991-0. Nat Med. 2022. PMID: 36109641 No abstract available.
References
-
- Ianiro G, et al. Incidence of bloodstream infections, length of hospital stay, and survival in patients with recurrent Clostridioides difficile infection treated with fecal microbiota transplantation or antibiotics: a prospective cohort study. Ann. Intern. Med. 2019;171:695–702. - PubMed
-
- Rossen NG, et al. Findings from a randomized controlled trial of fecal transplantation for patients with ulcerative colitis. Gastroenterology. 2015;149:110–118.e4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

