Hydropersulfides inhibit lipid peroxidation and ferroptosis by scavenging radicals
- PMID: 36109647
- PMCID: PMC7613997
- DOI: 10.1038/s41589-022-01145-w
Hydropersulfides inhibit lipid peroxidation and ferroptosis by scavenging radicals
Abstract
Ferroptosis is a type of cell death caused by radical-driven lipid peroxidation, leading to membrane damage and rupture. Here we show that enzymatically produced sulfane sulfur (S0) species, specifically hydropersulfides, scavenge endogenously generated free radicals and, thereby, suppress lipid peroxidation and ferroptosis. By providing sulfur for S0 biosynthesis, cysteine can support ferroptosis resistance independently of the canonical GPX4 pathway. Our results further suggest that hydropersulfides terminate radical chain reactions through the formation and self-recombination of perthiyl radicals. The autocatalytic regeneration of hydropersulfides may explain why low micromolar concentrations of persulfides suffice to produce potent cytoprotective effects on a background of millimolar concentrations of glutathione. We propose that increased S0 biosynthesis is an adaptive cellular response to radical-driven lipid peroxidation, potentially representing a primordial radical protection system.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







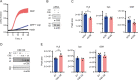
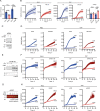






Comment in
-
A radical way to avoid cell death.Nat Chem Biol. 2023 Jan;19(1):1-2. doi: 10.1038/s41589-022-01174-5. Nat Chem Biol. 2023. PMID: 36280796 No abstract available.
References
-
- Dixon SJ, Stockwell BR. The hallmarks of ferroptosis. Annu. Rev. Cancer Biol. 2019;3:35–54. doi: 10.1146/annurev-cancerbio-030518-055844. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

