An intercellular transfer of telomeres rescues T cells from senescence and promotes long-term immunological memory
- PMID: 36109671
- PMCID: PMC7613731
- DOI: 10.1038/s41556-022-00991-z
An intercellular transfer of telomeres rescues T cells from senescence and promotes long-term immunological memory
Abstract
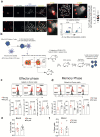
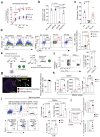
The common view is that T lymphocytes activate telomerase to delay senescence. Here we show that some T cells (primarily naïve and central memory cells) elongated telomeres by acquiring telomere vesicles from antigen-presenting cells (APCs) independently of telomerase action. Upon contact with these T cells, APCs degraded shelterin to donate telomeres, which were cleaved by the telomere trimming factor TZAP, and then transferred in extracellular vesicles at the immunological synapse. Telomere vesicles retained the Rad51 recombination factor that enabled telomere fusion with T-cell chromosome ends lengthening them by an average of ~3,000 base pairs. Thus, there are antigen-specific populations of T cells whose ageing fate decisions are based on telomere vesicle transfer upon initial contact with APCs. These telomere-acquiring T cells are protected from senescence before clonal division begins, conferring long-lasting immune protection.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Figures
















Comment in
-
Telomeres are a life-extending gift.Nat Cell Biol. 2022 Oct;24(10):1449-1450. doi: 10.1038/s41556-022-01004-9. Nat Cell Biol. 2022. PMID: 36168042 No abstract available.
-
Gift of life: APC to T cell telomere transfer.Nat Rev Immunol. 2022 Nov;22(11):653. doi: 10.1038/s41577-022-00790-5. Nat Rev Immunol. 2022. PMID: 36175503 No abstract available.
-
New mechanism to promote long-term T-cell immunity by telomere transfer from antigen-presenting cells.Cell Mol Immunol. 2023 Feb;20(2):117-118. doi: 10.1038/s41423-022-00949-z. Epub 2022 Nov 18. Cell Mol Immunol. 2023. PMID: 36400982 Free PMC article. No abstract available.
References
-
- Kipling D. Telomeres, replicative senescence and human ageing. Maturitas. 2001;38:25–37. discussion 37-8. - PubMed
-
- Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. - PubMed
-
- Blasco MA. Telomeres and human disease: ageing, cancer and beyond. Nat Rev Genet. 2005;6:611–22. - PubMed
-
- Cesare AJ, Reddel RR. Alternative lengthening of telomeres: models, mechanisms and implications. Nature Reviews Genetics. 2010;11:319–330. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

