Rapamycin and inulin for booster vaccine response stimulation (RIVASTIM)-rapamycin: study protocol for a randomised, controlled trial of immunosuppression modification with rapamycin to improve SARS-CoV-2 vaccine response in kidney transplant recipients
- PMID: 36109788
- PMCID: PMC9477178
- DOI: 10.1186/s13063-022-06634-w
Rapamycin and inulin for booster vaccine response stimulation (RIVASTIM)-rapamycin: study protocol for a randomised, controlled trial of immunosuppression modification with rapamycin to improve SARS-CoV-2 vaccine response in kidney transplant recipients
Abstract
Kidney transplant recipients are at an increased risk of severe COVID-19-associated hospitalisation and death. Vaccination has been a key public health strategy to reduce disease severity and infectivity, but the effectiveness of COVID vaccines is markedly reduced in kidney transplant recipients. Urgent strategies to enhance vaccine efficacy are needed.
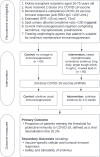
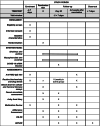
Methods: RIVASTIM-rapamycin is a multicentre, randomised, controlled trial examining the effect of immunosuppression modification prior to a third dose of COVID-19 vaccine in kidney transplant recipients who have failed to develop protective immunity to a 2-dose COVID-19 vaccine schedule. Participants will be randomised 1:1 to either remain on standard of care immunosuppression with tacrolimus, mycophenolate, and prednisolone (control) or cease mycophenolate and commence sirolimus (intervention) for 4 weeks prior to and following vaccination. The primary outcome is the proportion of participants in each trial arm who develop protective serological neutralisation of live SARS-CoV-2 virus at 4-6 weeks following a third COVID-19 vaccination. Secondary outcomes include SARS-CoV-receptor binding domain IgG, vaccine-specific immune cell populations and responses, and the safety and tolerability of sirolimus switch.
Discussion: Immunosuppression modification strategies may improve immunological vaccine response. We hypothesise that substituting the mTOR inhibitor sirolimus for mycophenolate in a triple drug regimen will enhance humoral and cell-mediated responses to COVID vaccination for kidney transplant recipients.
Trial registration: Australia New Zealand Clinical Trials Registry ACTRN12621001412820. Registered on 20 October 2021; https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=382891&isReview=true.
Keywords: COVID-19; Immunosuppression; Kidney transplantation; Randomised controlled trial; SARS-CoV-2; Sirolimus; Vaccination.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Rapamycin and inulin for third-dose vaccine response stimulation (RIVASTIM): Inulin - study protocol for a pilot, multicentre, randomised, double-blinded, controlled trial of dietary inulin to improve SARS-CoV-2 vaccine response in kidney transplant recipients.BMJ Open. 2022 Dec 1;12(12):e062747. doi: 10.1136/bmjopen-2022-062747. BMJ Open. 2022. PMID: 36456021 Free PMC article.
-
A randomized, double-blind, placebo-controlled phase III clinical trial to evaluate the efficacy and safety of SARS-CoV-2 vaccine (inactivated, Vero cell): a structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Apr 13;22(1):276. doi: 10.1186/s13063-021-05180-1. Trials. 2021. PMID: 33849629 Free PMC article.
-
Protocol for SARS-CoV-2 post-vaccine surveillance study in Australian adults and children with cancer: an observational study of safety and serological and immunological response to SARS-CoV-2 vaccination (SerOzNET).BMC Infect Dis. 2022 Jan 20;22(1):70. doi: 10.1186/s12879-021-07019-1. BMC Infect Dis. 2022. PMID: 35057745 Free PMC article.
-
Risk Factors for Weak Antibody Response of SARS-CoV-2 Vaccine in Adult Solid Organ Transplant Recipients: A Systemic Review and Meta-Analysis.Front Immunol. 2022 Jun 14;13:888385. doi: 10.3389/fimmu.2022.888385. eCollection 2022. Front Immunol. 2022. PMID: 35774786 Free PMC article.
-
Vaccination Against SARS-CoV-2 in Lung Transplant Recipients: Immunogenicity, Efficacy and Safety.Front Immunol. 2022 Jun 1;13:906225. doi: 10.3389/fimmu.2022.906225. eCollection 2022. Front Immunol. 2022. PMID: 35720376 Free PMC article. Review.
Cited by
-
Understanding and improving vaccine efficacy in older adults.Nat Aging. 2025 Aug;5(8):1455-1470. doi: 10.1038/s43587-025-00939-6. Epub 2025 Aug 14. Nat Aging. 2025. PMID: 40813812 Review.
-
Effects of Sirolimus Treatment on Fetal Hemoglobin Production and Response to SARS-CoV-2 Vaccination: A Case Report Study.Hematol Rep. 2023 Jul 12;15(3):432-439. doi: 10.3390/hematolrep15030044. Hematol Rep. 2023. PMID: 37489374 Free PMC article.
-
Humoral and cellular immunity to SARS-CoV-2 Ancestral and Omicron BA.5 variants following vaccination in myelofibrosis patients.Blood Cancer J. 2023 Apr 10;13(1):50. doi: 10.1038/s41408-023-00824-8. Blood Cancer J. 2023. PMID: 37032391 Free PMC article. No abstract available.
-
Rapamycin and inulin for third-dose vaccine response stimulation (RIVASTIM): Inulin - study protocol for a pilot, multicentre, randomised, double-blinded, controlled trial of dietary inulin to improve SARS-CoV-2 vaccine response in kidney transplant recipients.BMJ Open. 2022 Dec 1;12(12):e062747. doi: 10.1136/bmjopen-2022-062747. BMJ Open. 2022. PMID: 36456021 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

