Recent advances and limitations of mTOR inhibitors in the treatment of cancer
- PMID: 36109789
- PMCID: PMC9476305
- DOI: 10.1186/s12935-022-02706-8
Recent advances and limitations of mTOR inhibitors in the treatment of cancer
Abstract
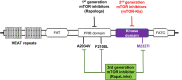
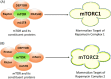
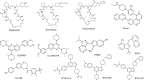
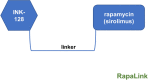
The PI3K-Akt-mechanistic (formerly mammalian) target of the rapamycin (mTOR) signaling pathway is important in a variety of biological activities, including cellular proliferation, survival, metabolism, autophagy, and immunity. Abnormal PI3K-Akt-mTOR signalling activation can promote transformation by creating a cellular environment conducive to it. Deregulation of such a system in terms of genetic mutations and amplification has been related to several human cancers. Consequently, mTOR has been recognized as a key target for the treatment of cancer, especially for treating cancers with elevated mTOR signaling due to genetic or metabolic disorders. In vitro and in vivo, rapamycin which is an immunosuppressant agent actively suppresses the activity of mTOR and reduces cancer cell growth. As a result, various sirolimus-derived compounds have now been established as therapies for cancer, and now these medications are being investigated in clinical studies. In this updated review, we discuss the usage of sirolimus-derived compounds and other drugs in several preclinical or clinical studies as well as explain some of the challenges involved in targeting mTOR for treating various human cancers.
Keywords: Cancer; Rapamycin; Targeted therapy; mTOR inhibitors; mTOR pathway; mTORC1; mTORC2.
© 2022. The Author(s).
Conflict of interest statement
Authors wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

