A longitudinal study on quality of life along the spectrum of Alzheimer's disease
- PMID: 36109800
- PMCID: PMC9476356
- DOI: 10.1186/s13195-022-01075-8
A longitudinal study on quality of life along the spectrum of Alzheimer's disease
Abstract
Background: Quality of life (QoL) is an important outcome from the perspective of patients and their caregivers, in both dementia and pre-dementia stages. Yet, little is known about the long-term changes in QoL over time. We aimed to compare the trajectories of QoL between amyloid-positive and amyloid-negative SCD or MCI patients and to evaluate QoL trajectories along the Alzheimer's disease (AD) continuum of cognitively normal to dementia.
Methods: We included longitudinal data of 447 subjective cognitive decline (SCD), 276 mild cognitive impairment (MCI), and 417 AD dementia patients from the Amsterdam Dementia Cohort. We compared QoL trajectories (EQ-5D and visual analog scale (VAS)) between (1) amyloid-positive and amyloid-negative SCD or MCI patients and (2) amyloid-positive SCD, MCI, and dementia patients with linear mixed-effect models. The models were adjusted for age, sex, Charlson Comorbidity Index (CCI), education, and EQ-5D scale (3 or 5 level).
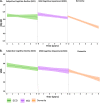
Results: In SCD, amyloid-positive participants had a higher VAS at baseline but showed a steeper decline over time in EQ-5D and VAS than amyloid-negative participants. Also, in MCI, amyloid-positive patients had higher QoL at baseline but subsequently showed a steeper decline in QoL over time compared to amyloid-negative patients. When we compared amyloid-positive patients along the Alzheimer continuum, we found no difference between SCD, MCI, or dementia in baseline QoL, but QoL decreased at a faster rate in the dementia stage compared with the of SCD and MCI stages.
Conclusions: QoL decreased at a faster rate over time in amyloid-positive SCD or MCI patients than amyloid-negative patients. QoL decreases over time along the entire AD continuum of SCD, MCI and dementia, with the strongest decrease in dementia patients. Knowledge of QoL trajectories is essential for the future evaluation of treatments in AD.
© 2022. The Author(s).
Conflict of interest statement
Arenda Mank, Ingrid S. van Maurik, Els D. Bakker and Johannes Berkhof report no financial disclosures or conflicts of interest.
Judith J.M. Rijnhart received a grant from the Amsterdam Public Health Research Institute, which was paid to the Amsterdam UMC.
Linus Jönsson has received consultancy fees from H.Lundbeck A/S.
Ron Handels reports the following related to this study: none. Ron Handels reports the following in the past 36 months outside this study: funding (paid to department) from Karolinska Institutet via affiliation, related to projects: SNAC (Sweden public funding 2016-2018), MIND-AD (public-private EU JPND grant 2017-2018), PRODEMOS (public EU H2020 2019-2023), SveDem (Sweden public-private collaboration 2019-2020), EUROFINGERS (public-private EU JPND; 2020-2023); grants (paid to department) from RECAGE H2020 (EU public funding; 2018-2022); grants (paid to department) from various ZonMw projects (NL public funding; 2017-2024); grants (paid to department) from patient association Alzheimer Nederland (NL fellowship; 2017-2019; WE.15-2016-09); grants (paid to department) from ROADMAP (IMI2; public-private collaboration; 2016-2019); consulting fees (paid to department) from institute for Medical Technology Assessment (advisory; 2021; content initiated by Biogen); consulting fees (paid to department) from Biogen Netherlands BV (advisory; 2021); consulting fees (paid to department) from Biogen MA Inc. (advisory; 2020); consulting fees (paid to department) from Eisai Inc. (advisory; 2019).
Charlotte E. Teunissen is supported by the European Commission (Marie Curie International Training Network, grant agreement No 860197 (MIRIADE), Innovative Medicines Initiatives 3TR (Horizon 2020, grant no 831434) EPND (IMI 2 Joint Undertaking (JU) under grant agreement No. 101034344 ) and JPND (bPRIDE), National MS Society (Progressive MS alliance) and Health Holland, the Dutch Research Council (ZonMW), Alzheimer Drug Discovery Foundation, The Selfridges Group Foundation, Alzheimer Netherlands, Alzheimer Association. CT is recipient of ABOARD, which is a public-private partnership receiving funding from ZonMW (#73305095007) and Health~Holland, Topsector Life Sciences & Health (PPP-allowance; #LSHM20106). ABOARD also receives funding from Edwin Bouw Fonds and Gieskes-Strijbisfonds
CET has a collaboration contract with ADx Neurosciences, Quanterix and Eli Lilly, performed contract research or received grants from AC-Immune, Axon Neurosciences, Bioconnect, Bioorchestra, Brainstorm Therapeutics, Celgene, EIP Pharma, Eisai, Grifols, Novo Nordisk, PeopleBio, Roche, Toyama, Vivoryon. She serves on editorial boards of Medidact Neurologie/Springer, Alzheimer Research and Therapy, Neurology: Neuroimmunology & Neuroinflammation, and is editor of a Neuromethods book Springer.
Bart van Berckel has received research support from EU-FP7, CTMM, ZonMw, NWO and Alzheimer Nederland. BvB has performed contract research for Rodin, IONIS, AVID, Eli Lilly, UCB, DIAN-TUI and Janssen. BvB was a speaker at a symposium organized by Springer Healthcare. BvB has a consultancy agreement with IXICO for the reading of PET scans. BvB is a trainer for GE. BvB only receives financial compensation from Amsterdam UMC.
Argonde van Harten was supported by funding from Alzheimer Netherlands, The Alzheimer Drug Discovery Foundation and the VUmc fund. Argonde van Harten has a collaboration contract with Quanterix corp.
Wiesje M. van der Flier: research programs of W.M. van der Flier have been funded by ZonMW, NWO, EU-FP7, EU-JPND, Alzheimer Nederland, Hersenstichting CardioVascular Onderzoek Nederland, Health~Holland, Topsector Life Sciences & Health, stichting Dioraphte, Gieskes-Strijbis fonds, stichting Equilibrio, Edwin Bouw fonds, Pasman stichting, stichting Alzheimer & Neuropsychiatrie Foundation, Philips, Biogen MA Inc., Novartis-NL, Life-MI, AVID, Roche BV, Fujifilm, Combinostics. WF holds the Pasman chair. WF is recipient of ABOARD, which is a public-private partnership receiving funding from ZonMW (#73305095007) and Health~Holland, Topsector Life Sciences & Health (PPP-allowance; #LSHM20106). WF has performed contract research for Biogen MA Inc., and Boehringer Ingelheim. WF has been an invited speaker at Boehringer Ingelheim, Biogen MA Inc., Danone, Eisai, WebMD Neurology (Medscape), Springer Healthcare. WF is consultant to Oxford Health Policy Forum CIC, Roche, and Biogen MA Inc. WF participated in advisory boards of Biogen MA Inc. and Roche. All funding is paid to her institution. WF was associate editor of Alzheimer, Research & Therapy in 2020/2021. WF is associate editor at Brain.
Figures

References
-
- Dementia: World Health Organization (WHO); 2021 [Available from: https://www.who.int/news-room/fact-sheets/detail/dementia.
-
- 2020 Alzheimer’s disease facts and figures. Alzheimer’s Dementia. 2020. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

