Systematic analysis of the varied designs of 819 therapeutic antibodies and Fc fusion proteins assigned international nonproprietary names
- PMID: 36109838
- PMCID: PMC9481088
- DOI: 10.1080/19420862.2022.2123299
Systematic analysis of the varied designs of 819 therapeutic antibodies and Fc fusion proteins assigned international nonproprietary names
Abstract
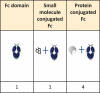
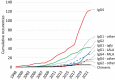
By combining and cross-checking data from the World Health Organization's lists of international nonproprietary names with three other databases, we assembled a dataset of amino acid sequences of 819 antibody-related therapeutics. This enabled the systematic tabulation of 57 different molecular formats and about 90 different constant-region variants (47 for silencing, 14 for enhancement, 8 for modifying binding to FcRn, 13 for heterodimerization, 4 for site-specific modification and 4 for stabilization), as well as the frequencies of different targets and immunoglobulin allotypes. The curated dataset provides a resource for researchers, giving insights into trends in antibody engineering and a guide to the most frequently tested designs.
Keywords: Allotype; FC receptor; FC region; immunoglobulin fusion protein; immunoglobulin sequence; international nonproprietary name; therapeutic antibody.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures







References
-
- World Health Organisation. Geneva, Switzerland. 2014. https://www.who.int/publications/i/item/who-emp-rht-tsn-2019-1.
-
- World Health Organisation. Switzerland, Geneva. 2017. https://cdn.who.int/media/docs/default-source/international-nonproprieta....
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
