Fine-scale genetic structure and wolbachia infection of aedes albopictus (Diptera: Culicidae) in Nanjing city, China
- PMID: 36110209
- PMCID: PMC9468874
- DOI: 10.3389/fgene.2022.827655
Fine-scale genetic structure and wolbachia infection of aedes albopictus (Diptera: Culicidae) in Nanjing city, China
Abstract
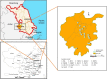
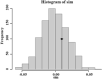
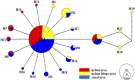
Background: Aedes albopictus is an indigenous primary vector of dengue and Zika viruses in China. Wolbachia is a gram-negative and common intracellular bacteria, which is maternally inherited endosymbionts and could expand their propagation in host populations by means of various manipulations. Compared with research on the dispersion of Ae. albopictus at the macrospatial level (mainly at the country or continent level), little is known about its variation and Wolbachia infection at the microspatial level, which is essential for its management. Meanwhile, no local cases of dengue fever have been recorded in the history of Nanjing, which implies that few adulticides have been applied in the city. Thus, the present study examines how the Ae. albopictus population varies and the Wolbachia infection status of each population among microspatial regions of Nanjing City. Methods: The genetic structure of 17 Aedes albopictus populations collected from urban, urban fringe, and rural regions of Nanjing City was investigated based on 9 microsatellite loci and the mitochondrial coxI gene. The Wolbachia infection status of each population was also assessed with Wolbachia A- and Wolbachia B-specific primers. Results: Nine out of 58 tested pairs of microsatellite markers were highly polymorphic, with a mean PIC value of 0.560, and these markers were therefore chosen for microsatellite genotyping analysis. The Na value of each Ae. albopictus population was very high, and the urban area populations (7.353 ± 4.975) showed a lower mean value than the urban fringe region populations (7.866 ± 5.010). A total of 19 coxI haplotypes were observed among 329 Ae. albopictus individuals via haplotype genotyping, with the highest diversity observed among the urban fringe Ae. albopictus populations (Hd = 0.456) and the lowest among the urban populations (Hd = 0.277). Each Ae. albopictus population showed significant departure from HWE, and significant population expansion was observed in only three populations from the urban (ZSL), urban fringe (HAJY), and rural areas (HSZY) (p < 0.05). Combined with DAPC analysis, all the Ae. albopictus populations were adequately allocated to two clades with significant genetic differences according to population structure analysis, and the best K value was equal to two. AMOVA results showed that most (96.18%) of the genetic variation detected in Ae. albopictus occurred within individuals (FIT = 0.22238, p < 0.0001), while no significant positive correlation was observed via isolation by distance (IBD) analysis (R 2 = 0.03262, p = 0.584). The TCS network of all haplotypes showed that haplotype 1 (H1) and haplotype 4 (H4) were the most frequent haplotypes among all populations, and the haplotype frequency significantly increased from urban regions (36.84%) to rural regions (68.42%). Frequent migration was observed among Ae. albopictus populations from rural to urban regions via the urban fringe region, with four direct migration routes between rural and urban regions. Furthermore, Wolbachia genotyping results showed that most of the individuals of each population were coinfected with Wolbachia A and Wolbachia B. The independent infection rate of Wolbachia A was slightly higher than that of Wolbachia B, and no significant differences were observed among different regions. Conclusion: In the microspatial environment of Nanjing City, the urban fringe region is an important region for the dispersion of Ae. albopictus populations between rural and urban areas, and Wolbachia A and Wolbachia B coinfection is the most common Wolbachia infection status in all Ae. albopictus populations among different regions.
Keywords: Aedes albopictus; genetic structure; haplotype; microsatellite loci; microspatial; urban region; wolbachia.
Copyright © 2022 Zhang, Gao, Xing, Guo, Li, Dong, Zheng, Ma, Wu, Zhu, Zhao, Liu, Yan, Chu and Zhao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Dispersal patterns and population genetic structure of Aedes albopictus (Diptera: Culicidae) in three different climatic regions of China.Parasit Vectors. 2021 Jan 6;14(1):12. doi: 10.1186/s13071-020-04521-4. Parasit Vectors. 2021. PMID: 33407824 Free PMC article.
-
Genetic structure and Wolbachia genotyping in naturally occurring populations of Aedes albopictus across contiguous landscapes of Orissa, India.PLoS One. 2014 Apr 8;9(4):e94094. doi: 10.1371/journal.pone.0094094. eCollection 2014. PLoS One. 2014. PMID: 24714653 Free PMC article.
-
Patterns of spatial genetic structures in Aedes albopictus (Diptera: Culicidae) populations in China.Parasit Vectors. 2019 Nov 21;12(1):552. doi: 10.1186/s13071-019-3801-4. Parasit Vectors. 2019. PMID: 31752961 Free PMC article.
-
Molecular evidence for new sympatric cryptic species of Aedes albopictus (Diptera: Culicidae) in China: A new threat from Aedes albopictus subgroup?Parasit Vectors. 2018 Apr 4;11(1):228. doi: 10.1186/s13071-018-2814-8. Parasit Vectors. 2018. PMID: 29618379 Free PMC article.
-
Critical review of the vector status of Aedes albopictus.Med Vet Entomol. 2004 Sep;18(3):215-27. doi: 10.1111/j.0269-283X.2004.00513.x. Med Vet Entomol. 2004. PMID: 15347388 Review.
Cited by
-
Population genetic characteristics of Aedes aegypti in 2019 and 2020 under the distinct circumstances of dengue outbreak and the COVID-19 pandemic in Yunnan Province, China.Front Genet. 2023 Mar 9;14:1107893. doi: 10.3389/fgene.2023.1107893. eCollection 2023. Front Genet. 2023. PMID: 36968606 Free PMC article.
-
Genetic Diversity and Population Genetic Structure of Aedes albopictus in the Yangtze River Basin, China.Genes (Basel). 2022 Oct 26;13(11):1950. doi: 10.3390/genes13111950. Genes (Basel). 2022. PMID: 36360187 Free PMC article.
-
Population genetic structure of Culex tritaeniorhynchus in different types of climatic zones in China.BMC Genomics. 2024 Jul 5;25(1):673. doi: 10.1186/s12864-024-10589-4. BMC Genomics. 2024. PMID: 38969975 Free PMC article.
References
-
- Baolin L. (1990). Dengue vector and dengue control in China: Guizhou People. Guizhou: s Publishing House.
-
- Beebe N. W., Ambrose L., Hill L. A., Davis J. B., Hapgood G., Cooper R. D., et al. (2013). Tracing the tiger: Population genetics provides valuable insights into the Aedes (stegomyia) albopictus invasion of the australasian region. PLoS Negl. Trop. Dis. 7 (8), e2361. 10.1371/journal.pntd.0002361 - DOI - PMC - PubMed
-
- Bonacum J., DeSalle R., O’Grady P., Olivera D., Wintermute J., Zilversmit M. J. D. I. S. (2001). New nuclear and mitochondrial primers for systematics and comparative genomics in Drosophilidae. Dros. Inf. Serv. 84, 201–204.
LinkOut - more resources
Full Text Sources

