Genetic engineering for enhanced production of a novel alkaline protease BSP-1 in Bacillus amyloliquefaciens
- PMID: 36110310
- PMCID: PMC9468883
- DOI: 10.3389/fbioe.2022.977215
Genetic engineering for enhanced production of a novel alkaline protease BSP-1 in Bacillus amyloliquefaciens
Abstract
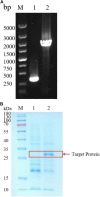
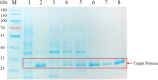
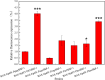
Alkaline protease has been widely applied in food, medicine, environmental protection and other industrial fields. However, the current activity and yield of alkaline protease cannot meet the demand. Therefore, it is important to identify new alkaline proteases with high activity. In this study, we cloned a potential alkaline protease gene bsp-1 from a Bacillus subtilis strain isolated in our laboratory. BSP-1 shows the highest sequence similarity to subtilisin NAT (S51909) from B. subtilis natto. Then, we expressed BSP-1 in Bacillus amyloliquefaciens BAX-9 and analyzed the protein expression level under a collection of promoters. The results show that the P43 promoter resulted in the highest transcription level, protein level and enzyme activity. Finally, we obtained a maximum activity of 524.12 U/mL using the P43 promoter after fermentation medium optimization. In conclusion, this study identified an alkaline protease gene bsp-1 from B. subtilis and provided a new method for high-efficiency alkaline protease expression in B. amyloliquefaciens.
Keywords: alkaline protease; bacillus amyloliquefaciens; fermentation optimization; promoter screening; recombinant expression.
Copyright © 2022 Jiang, Ye, Liu, Huang, Jiang, Zou, Li, Han and Wei.
Conflict of interest statement
YL was employed by GeneMind Biosciences Company Limited. CY and KH were employed by China National Tobacco Corporation. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Agrebi R., Haddar A., Hmidet N., Jellouli K., Manni L., Nasri M. (2009). BSF1 fibrinolytic enzyme from a marine bacterium Bacillus subtilis A26: Purification, biochemical and molecular characterization. Process Biochem. 44 (11), 1252–1259. 10.1016/j.procbio.2009.06.024 - DOI
LinkOut - more resources
Full Text Sources
Research Materials

