Therapeutic Effects of Silibinin Against Polycystic Ovary Syndrome Induced by Letrozole in Rats via Its Potential Anti-Inflammatory and Anti-Oxidant Activities
- PMID: 36110507
- PMCID: PMC9469941
- DOI: 10.2147/JIR.S379725
Therapeutic Effects of Silibinin Against Polycystic Ovary Syndrome Induced by Letrozole in Rats via Its Potential Anti-Inflammatory and Anti-Oxidant Activities
Abstract
Background: Current therapies for polycystic ovary syndrome (PCOS) are accompanied by unwanted effects. Silibinin; a flavonolignan has pleiotropic activities and favorable safety profile.
Purpose: To investigate the efficacy of silibinin on estrous cyclicity, inflammation, oxidative stress and ovarian morphology in letrozole-induced PCOS in rats.
Methods: Forty-eight female Wistar albino rats were divided into 2 sets. Rats of the first set (n = 12), assigned as a negative control (NC) received only the vehicle, rats of the second set (n = 36), assigned as PCOS rats, were given letrozole 1mg/Kg orally for 21 days. On day 21, six rats from the first set and six rats from the second set were euthanized for confirmation of PCOS-induction. The remaining animals from the first set assigned as group 1, those in the second set (n = 30) were equally divided into 5 groups and treated daily for 19 days as follows: group 2 (positive control) received only the vehicle, group 3 treated with metformin 300mg/Kg orally, groups 4 and 5 treated respectively with 100 and 200 mg/Kg silibinin intraperitoneally (IP), and group 6 treated with a combination of metformin 300mg/Kg orally and silibinin 100mg/Kg IP. On day 40, blood samples were examined for luteinizing hormone (LH), testosterone (TS) and estradiol (EST) levels, the anti-inflammatory and antioxidant parameters, ovarian and uterine morphology.
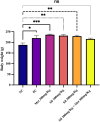
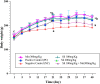
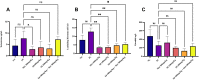
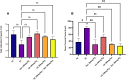
Results: Silibinin alone or in combination with metformin was found to be effective in restoring the regularity of estrous cycle by ameliorating the abnormal alterations of LH, TS, EST, tumor necrosis factor (TNF)-α, and oxidative status and by resuming the appearance of corpora lutea and decreasing or even total absence of cystic follicles in the ovaries.
Conclusion: Silibinin was effective in restoring estrous regularities and alleviating hormonal and histomorphological abnormalities of the ovarian and uterine tissues, this could be due to its anti-androgenic, anti-inflammatory and antioxidant properties.
Keywords: PCOS; estrous cycle; inflammation; silybin; testosterone.
© 2022 Marouf et al.
Conflict of interest statement
The authors report no conflicts of interest in this work
Figures





Similar articles
-
Effect of Silibinin on Dyslipidemia and Glycemic Alteration Associated with Polycystic Ovarian Syndrome: An Experimental Study on Rats.Diabetes Metab Syndr Obes. 2022 Sep 7;15:2771-2780. doi: 10.2147/DMSO.S377404. eCollection 2022. Diabetes Metab Syndr Obes. 2022. PMID: 36105429 Free PMC article.
-
Therapeutic potentials of Quercetin in management of polycystic ovarian syndrome using Letrozole induced rat model: a histological and a biochemical study.J Ovarian Res. 2018 Apr 3;11(1):26. doi: 10.1186/s13048-018-0400-5. J Ovarian Res. 2018. PMID: 29615083 Free PMC article.
-
Promising drug candidates for the treatment of polycystic ovary syndrome (PCOS) as alternatives to the classical medication metformin.Eur J Pharmacol. 2023 Dec 5;960:176119. doi: 10.1016/j.ejphar.2023.176119. Epub 2023 Oct 16. Eur J Pharmacol. 2023. PMID: 37852569
-
Partially purified non-polar phytocomponents from Aloe barbadensis Mill. gel restores metabolic and reproductive comorbidities in letrozole-induced polycystic ovary syndrome rodent model- an "in-vivo" study.J Ethnopharmacol. 2022 Jun 12;291:115161. doi: 10.1016/j.jep.2022.115161. Epub 2022 Mar 7. J Ethnopharmacol. 2022. PMID: 35271948
-
Pathophysiological changes in experimental polycystic ovary syndrome in female albino rats: Using either hemin or L-arginine.J Cell Physiol. 2019 Jun;234(6):8426-8435. doi: 10.1002/jcp.27757. Epub 2018 Nov 15. J Cell Physiol. 2019. PMID: 30443939 Review.
Cited by
-
Suppression of neurotransmission on gonadotropin-releasing hormone neurons in letrozole-induced polycystic ovary syndrome: A mouse model.Front Endocrinol (Lausanne). 2023 Jan 9;13:1059255. doi: 10.3389/fendo.2022.1059255. eCollection 2022. Front Endocrinol (Lausanne). 2023. PMID: 36699037 Free PMC article.
-
Evidence for causal effects of polycystic ovary syndrome on oxidative stress: a two-sample mendelian randomisation study.BMC Med Genomics. 2023 Jun 19;16(1):141. doi: 10.1186/s12920-023-01581-0. BMC Med Genomics. 2023. PMID: 37337194 Free PMC article.
-
Multitargeted Effects of Plantago ovata Ethanol Extract in Experimental Rat Streptozotocin-Induced Diabetes Mellitus and Letrozole-Induced Polycystic Ovary Syndrome.Int J Mol Sci. 2025 May 14;26(10):4712. doi: 10.3390/ijms26104712. Int J Mol Sci. 2025. PMID: 40429855 Free PMC article.
-
Efficacy and Mechanisms of Silybum Marianum, Silymarin, and Silibinin on Rheumatoid Arthritis and Osteoarthritis Symptoms: A Systematic Review.Curr Rheumatol Rev. 2024;20(4):414-425. doi: 10.2174/0115733971266397231122080247. Curr Rheumatol Rev. 2024. PMID: 38314596
-
Natural compounds in the management of polycystic ovary syndrome: a comprehensive review of hormonal regulation and therapeutic potential.Front Nutr. 2025 Feb 11;12:1520695. doi: 10.3389/fnut.2025.1520695. eCollection 2025. Front Nutr. 2025. PMID: 40008316 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials

