Linezolid resistance in multidrug-resistant mycobacterium tuberculosis: A systematic review and meta-analysis
- PMID: 36110536
- PMCID: PMC9468755
- DOI: 10.3389/fphar.2022.955050
Linezolid resistance in multidrug-resistant mycobacterium tuberculosis: A systematic review and meta-analysis
Abstract
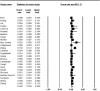
Introduction: Linezolid (LNZ) is an effective antibiotic to treat patients with multidrug-resistant tuberculosis (MDR-TB) treatment failure. M. tuberculosis strains resistant to isoniazid and rifampin are defined as MDR-TB. In recent years, resistance to LNZ among MDR-TB cases has been reported in several different countries. In this study, we performed a systematic review and meta-analysis to investigate the prevalence of LNZ resistance among MDR-TB isolates. Methods: The databases of Embase, PubMed/Medline, and Web of Science were searched systematically from January 2000 to April 2021. Statistical analyses were performed by using Comprehensive Meta-Analysis software. Heterogeneity was reported by using the t-squared statistic and Q-statistic. Begg's rank correlation in combination with the funnel plot were used to evaluate any possible publication bias. Results: In total, 25 studies were selected for meta-analysis from 14 different countries; the majority was from China (n = 5) and Turkey (n = 4). Moreover, 7,366 patients were infected with MDR M. tuberculosis. Among the study population, 98 patients were co-infected with HIV, and 18 patients with hepatitis C virus (HCV). Furthermore, 28 cases had diabetes, and139 cases were alcohol abuser. Overall, 4,956 MDR M. tuberculosis strains were isolated from TB patients. The pooled frequency of LNZ resistance among the clinical isolates of MDR M. tuberculosis was 4.2% (95%). Begg's (p = 0.72) test showed no evidence of publication bias. Conclusion: LNZ resistance among MDR M. tuberculosis isolates is increasing. On the other hand, long-term treatment of MDR-TB cases with LNZ alone is associated with several adverse effects. Thus, it is recommended that newer anti-TB drugs, including bedaquiline and delamanid, in combination with linezolid could increase its effectiveness and decrease toxicities. However, more studies should be done in this field.
Keywords: MDR-TB; TB; linezolid; meta-analysis; resistance; tuberculosis.
Copyright © 2022 Azimi, Khoshnood, Asadi, Heidary, Mahmoudi, Kaviar, Hallajzadeh and Nasiri.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Ahmed I., Jabeen K., Inayat R., Hasan R. (2013). Susceptibility testing of extensively drug-resistant and pre-extensively drug-resistant Mycobacterium tuberculosis against levofloxacin, linezolid, and amoxicillin-clavulanate. Antimicrob. Agents Chemother. 57 (6), 2522–2525. 10.1128/AAC.02020-12 - DOI - PMC - PubMed
-
- Alcalá L., Ruiz-Serrano M. J., Turégano C. P-F., De Viedma D. G., Díaz-Infantes M., Marin-Arriaza M., et al. (2003). In vitro activities of linezolid against clinical isolates of Mycobacterium tuberculosis that are susceptible or resistant to first-line antituberculous drugs. Antimicrob. Agents Chemother. 47 (1), 416–417. 10.1128/aac.47.1.416-417.2003 - DOI - PMC - PubMed
-
- Azimi T., Shariati A., Fallah F., Imani Fooladi A. A., Hashemi A., Goudarzi H., et al. (2017). Mycobacterium tuberculosis genotyping using MIRU-VNTR typing. J. Mazandaran Univ. Med. Sci. 27 (149), 40–48.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

