Pulmonary adverse events associated with hypertension in non-small cell lung cancer patients receiving PD-1/PD-L1 inhibitors
- PMID: 36110543
- PMCID: PMC9468816
- DOI: 10.3389/fphar.2022.944342
Pulmonary adverse events associated with hypertension in non-small cell lung cancer patients receiving PD-1/PD-L1 inhibitors
Abstract
Introduction: Non-small cell lung cancer patients have gained therapeutic benefits from immune checkpoint inhibitors, although immune-related adverse events (irAEs) could be inevitable. Whether irAEs are associated with chronic diseases is still unclear, our study aims to clarify the distinct adverse events in NSCLC patients with concomitant hypertension. Methods: Adverse event cases were searched and collected in the Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS) database from January 2015 to December 2021. We performed disproportionality analysis to detect safety signals by calculating reporting odds ratios (ROR) and corresponding 95% confidence intervals (95% CIs), information component (IC), and the lower bound of the information component 95% credibility interval (IC025). Results: Among 17,163 NSCLC patients under treatment with single-agent anti-programmed death-1/programmed death ligand-1 (PD-1/PD-L1) inhibitor (nivolumab, pembrolizumab, cemiplimab, durvalumab, atezolizumab, and avelumab), 497 patients had hypertension while 16,666 patients had no hypertension. 4,283 pulmonary AEs were reported, including 166 patients with hypertension and 4,117 patients without hypertension. Compared with patients without hypertension, patients with hypertension were positively associated with increased reporting of interstitial lung disease (ROR = 3.62, 95%CI 2.68-4.89, IC = 1.54, IC025 = 0.57) among patients receiving anti-PD-1 treatment. The median duration of onset from the time of initiation of anti-PD-1 administration was 28 days (IQR, 12.00-84.25). Conclusion: Our pharmacovigilance analysis showed the profile of pulmonary toxicities in NSCLC patients with hypertension caused by anti-PD-1/PD-L1 inhibitors. Interstitial lung disease was the statistically significant reporting adverse event in patients with hypertension receiving anti-PD-1 treatment.
Keywords: FAERS; NSCLC; hypertension; immune checkpoint inhibitor; pharmacovigilance.
Copyright © 2022 Chen, Wen, Chu, Liu and Su.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
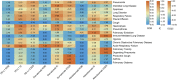
Figures



Similar articles
-
Cardiovascular adverse events and immune-related adverse events associated with PD-1/PD-L1 inhibitors for head and neck squamous cell carcinoma (HNSCC).Sci Rep. 2024 Oct 29;14(1):25919. doi: 10.1038/s41598-024-75099-5. Sci Rep. 2024. PMID: 39472591 Free PMC article.
-
Pulmonary Immune-Related Adverse Events of PD-1 Versus PD-L1 Checkpoint Inhibitors: A Retrospective Review of Pharmacovigilance.J Immunother Precis Oncol. 2023 Dec 2;6(4):177-184. doi: 10.36401/JIPO-22-38. eCollection 2023 Nov. J Immunother Precis Oncol. 2023. PMID: 38143955 Free PMC article.
-
Pharmacovigilance study on the reporting frequency of atrial fibrillation with immune checkpoint inhibitors: insights from FDA Adverse Event Reporting System.Ther Adv Drug Saf. 2025 Apr 21;16:20420986241312497. doi: 10.1177/20420986241312497. eCollection 2025. Ther Adv Drug Saf. 2025. PMID: 40290514 Free PMC article.
-
PD-1/PD-L1 inhibitor-induced immune thrombocytopenia: A pharmacovigilance study and systematic review.Int Immunopharmacol. 2024 Mar 10;129:111606. doi: 10.1016/j.intimp.2024.111606. Epub 2024 Feb 14. Int Immunopharmacol. 2024. PMID: 38359661
-
Immune-related adverse events associated with programmed cell death protein-1 and programmed cell death ligand 1 inhibitors for non-small cell lung cancer: a PRISMA systematic review and meta-analysis.BMC Cancer. 2019 Jun 10;19(1):558. doi: 10.1186/s12885-019-5701-6. BMC Cancer. 2019. PMID: 31182061 Free PMC article.
Cited by
-
Drug-induced interstitial lung disease: a real-world pharmacovigilance study of the FDA Adverse Event Reporting System from 2004 to 2021.Ther Adv Drug Saf. 2024 Jan 27;15:20420986231224227. doi: 10.1177/20420986231224227. eCollection 2024. Ther Adv Drug Saf. 2024. PMID: 38293566 Free PMC article.
-
Comparative analysis of adverse event risks in breast cancer patients receiving pembrolizumab combined with paclitaxel versus paclitaxel monotherapy: insights from the FAERS database.Front Pharmacol. 2024 Aug 21;15:1345671. doi: 10.3389/fphar.2024.1345671. eCollection 2024. Front Pharmacol. 2024. PMID: 39234109 Free PMC article.
-
A disproportionality analysis of adverse events associated to pertuzumab in the FDA Adverse Event Reporting System (FAERS).BMC Pharmacol Toxicol. 2023 Nov 13;24(1):62. doi: 10.1186/s40360-023-00702-w. BMC Pharmacol Toxicol. 2023. PMID: 37957717 Free PMC article.
-
Comparisons of adverse events associated with immune checkpoint inhibitors in the treatment of non-small cell lung cancer: a real-world disproportionality analysis based on the FDA adverse event reporting system.BMC Cancer. 2025 Feb 7;25(1):216. doi: 10.1186/s12885-025-13614-1. BMC Cancer. 2025. PMID: 39920614 Free PMC article.
References
-
- Alexander M. R., Norlander A. E., Elijovich F., Atreya R. V., Gaye A., Gnecco J. S., et al. (2019). Human monocyte transcriptional profiling identifies IL-18 receptor accessory protein and lactoferrin as novel immune targets in hypertension. Br. J. Pharmacol. 176, 2015–2027. 10.1111/bph.14364 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

