CRISPR/Cas9 based blockade of IL-10 signaling impairs lipid and tissue homeostasis to accelerate atherosclerosis
- PMID: 36110841
- PMCID: PMC9469689
- DOI: 10.3389/fimmu.2022.999470
CRISPR/Cas9 based blockade of IL-10 signaling impairs lipid and tissue homeostasis to accelerate atherosclerosis
Abstract
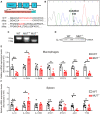
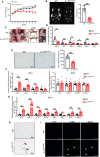
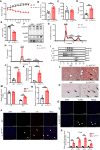
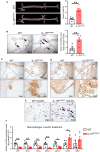
Interleukin-10 (IL-10) is a widely recognized immunosuppressive factor. Although the concept that IL-10 executes an anti-inflammatory role is accepted, the relationship between IL-10 and atherosclerosis is still unclear, thus limiting the application of IL-10-based therapies for this disease. Emerging evidence suggests that IL-10 also plays a key role in energy metabolism and regulation of gut microbiota; however, whether IL-10 can affect atherosclerotic lesion development by integrating lipid and tissue homeostasis has not been investigated. In the present study, we developed a human-like hamster model deficient in IL-10 using CRISPR/Cas9 technology. Our results showed that loss of IL-10 changed the gut microbiota in hamsters on chow diet, leading to an increase in lipopolysaccharide (LPS) production and elevated concentration of LPS in plasma. These changes were associated with systemic inflammation, lipodystrophy, and dyslipidemia. Upon high cholesterol/high fat diet feeding, IL-10-deficient hamsters exhibited abnormal distribution of triglyceride and cholesterol in lipoprotein particles, impaired lipid transport in macrophages and aggravated atherosclerosis. These findings show that silencing IL-10 signaling in hamsters promotes atherosclerosis by affecting lipid and tissue homeostasis through a gut microbiota/adipose tissue/liver axis.
Keywords: CRISPR/Cas9; Syrian golden hamster; atherosclerosis; gut microbiota; interleukin-10.
Copyright © 2022 Shi, Guo, Yu, Hou, Liu, Gao, Wei, Zhang, Huang, Wang, Liu, Tontonoz and Xian.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: A report of the American college of Cardiology/American heart association task force on clinical practice guidelines. Circulation (2019) 140(11):e596–646. doi: 10.1161/CIR.0000000000000725 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

