An optimized Factor H-Fc fusion protein against multidrug-resistant Neisseria gonorrhoeae
- PMID: 36110842
- PMCID: PMC9468773
- DOI: 10.3389/fimmu.2022.975676
An optimized Factor H-Fc fusion protein against multidrug-resistant Neisseria gonorrhoeae
Abstract
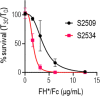
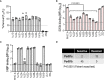
Novel therapeutics against the global threat of multidrug-resistant Neisseria gonorrhoeae are urgently needed. Gonococci evade killing by complement by binding factor H (FH), a key inhibitor of the alternative pathway. FH comprises 20 short consensus repeat (SCR) domains organized as a single chain. Gonococci bind FH through domains 6 and 7, and C-terminal domains 18 through 20. Previously, we showed that a chimeric protein comprising (from the N- to C-terminus) FH domains 18-20 (containing a point mutation in domain 19 to prevent lysis of host cells) fused to human IgG1 Fc (called FH*/Fc1) killed gonococci in a complement-dependent manner and reduced the duration and bacterial burden in the mouse vaginal colonization model of gonorrhea. Considering the N. gonorrhoeae-binding FH domains 18-20 are C-terminal in native FH, we reasoned that positioning Fc N-terminal to FH* (Fc1/FH*) would improve binding and bactericidal activity. Although both molecules bound gonococci similarly, Fc1/FH* displayed a 5-fold lower IC50 (the concentration required for 50% killing in complement-dependent bactericidal assays) than FH*/Fc1. To further increase complement activation, we replaced human IgG1 Fc in Fc1/FH* with Fc from human IgG3, the most potent complement-activating IgG subclass, to obtain Fc3/FH*. Bactericidal activity was further increased ~2.3-fold in Fc3/FH* compared to Fc1/FH*. Fc3/FH* killed (defined by <50% survival) 45/45 (100%) diverse PorB1B-expessing gonococci, but only 2/15 PorB1A-expressing isolates, in a complement-dependent manner. Decreased Fc3/FH* binding accounted for the limited activity against PorB1A strains. Fc3/FH* was efficacious against all four tested PorB1B gonococcal strains in the mouse vaginal colonization model when administered at a dose of 5 µg intravaginally, daily. Furthermore, Fc3/FH* retained bactericidal activity when reconstituted following lyophilization or spray-drying, suggesting feasibility for formulation into intravaginal rings. In conclusion, Fc3/FH* represents a promising prophylactic immunotherapeutic against multidrug-resistant gonococci.
Keywords: Fc fusion protein; Neisseria gonorrhoeae; Nicotiana benthamiana; complement; factor H; gonorrhea; immunotherapeutic.
Copyright © 2022 Shaughnessy, Chabeda, Tran, Zheng, Nowak, Steffens, DeOliveira, Gulati, Lewis, Maclean, Moss, Wycoff and Ram.
Conflict of interest statement
Authors YT, JM, and KW were employed by Planet Biotechnology, Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


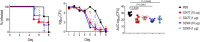


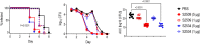
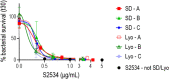
References
-
- Cohen MS, Hoffman IF, Royce RA, Kazembe P, Dyer JR, Daly CC, et al. . Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. AIDSCAP Malawi research group. Lancet (1997) 349:1868–73. doi: 10.1016/S0140-6736(97)02190-9 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

