Examination of the role of necroptotic damage-associated molecular patterns in tissue fibrosis
- PMID: 36110858
- PMCID: PMC9468929
- DOI: 10.3389/fimmu.2022.886374
Examination of the role of necroptotic damage-associated molecular patterns in tissue fibrosis
Erratum in
-
Corrigendum: Examination of the role of necroptotic damage-associated molecular patterns in tissue fibrosis.Front Immunol. 2022 Nov 9;13:1048026. doi: 10.3389/fimmu.2022.1048026. eCollection 2022. Front Immunol. 2022. PMID: 36439139 Free PMC article.
Abstract
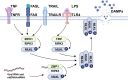
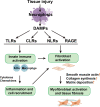
Fibrosis is defined as the abnormal and excessive deposition of extracellular matrix (ECM) components, which leads to tissue or organ dysfunction and failure. However, the pathological mechanisms underlying fibrosis remain unclear. The inflammatory response induced by tissue injury is closely associated with tissue fibrosis. Recently, an increasing number of studies have linked necroptosis to inflammation and fibrosis. Necroptosis is a type of preprogrammed death caused by death receptors, interferons, Toll-like receptors, intracellular RNA and DNA sensors, and other mediators. These activate receptor-interacting protein kinase (RIPK) 1, which recruits and phosphorylates RIPK3. RIPK3 then phosphorylates a mixed lineage kinase domain-like protein and causes its oligomerization, leading to rapid plasma membrane permeabilization, the release of cellular contents, and exposure of damage-associated molecular patterns (DAMPs). DAMPs, as inflammatory mediators, are involved in the loss of balance between extensive inflammation and tissue regeneration, leading to remodeling, the hallmark of fibrosis. In this review, we discuss the role of necroptotic DAMPs in tissue fibrosis and highlight the inflammatory responses induced by DAMPs in tissue ECM remodeling. By summarizing the existing literature on this topic, we underscore the gaps in the current research, providing a framework for future investigations into the relationship among necroptosis, DAMPs, and fibrosis, as well as a reference for later transformation into clinical treatment.
Keywords: DAMPs; RIPK3; fibrosis; inflammation; necroptosis.
Copyright © 2022 Liu, Lu and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Novel Roles of Necroptosis Mediator Receptor-Interacting Protein Kinase 3 in Kidney Injury.Nephron. 2022;146(3):259-263. doi: 10.1159/000517732. Epub 2021 Jul 20. Nephron. 2022. PMID: 34284405 Free PMC article. Review.
-
Necroptotic cell death in anti-cancer therapy.Immunol Rev. 2017 Nov;280(1):207-219. doi: 10.1111/imr.12583. Immunol Rev. 2017. PMID: 29027225 Review.
-
A FRET biosensor for necroptosis uncovers two different modes of the release of DAMPs.Nat Commun. 2018 Oct 26;9(1):4457. doi: 10.1038/s41467-018-06985-6. Nat Commun. 2018. PMID: 30367066 Free PMC article.
-
The Inflammatory Signal Adaptor RIPK3: Functions Beyond Necroptosis.Int Rev Cell Mol Biol. 2017;328:253-275. doi: 10.1016/bs.ircmb.2016.08.007. Epub 2016 Sep 22. Int Rev Cell Mol Biol. 2017. PMID: 28069136 Free PMC article. Review.
-
Complex roles of necroptosis in cancer.J Zhejiang Univ Sci B. 2019 May;20(5):399-413. doi: 10.1631/jzus.B1900160. J Zhejiang Univ Sci B. 2019. PMID: 31090266 Free PMC article. Review.
Cited by
-
Regulated cell death and DAMPs as biomarkers and therapeutic targets in normothermic perfusion of transplant organs. Part 1: their emergence from injuries to the donor organ.Front Transplant. 2025 Apr 24;4:1571516. doi: 10.3389/frtra.2025.1571516. eCollection 2025. Front Transplant. 2025. PMID: 40343197 Free PMC article. Review.
-
Programmed cell revival from imminent cell death enhances tissue repair and regeneration.EMBO J. 2025 Aug 21. doi: 10.1038/s44318-025-00540-y. Online ahead of print. EMBO J. 2025. PMID: 40841709
-
Identification of TNFRSF1A as a potential biomarker for osteosarcoma.Cancer Biomark. 2024;39(4):299-312. doi: 10.3233/CBM-230086. Cancer Biomark. 2024. PMID: 38250759 Free PMC article.
-
Ferroptosis in idiopathic pulmonary fibrosis: mechanisms, impact, and therapeutic opportunities.Front Immunol. 2025 May 21;16:1567994. doi: 10.3389/fimmu.2025.1567994. eCollection 2025. Front Immunol. 2025. PMID: 40469306 Free PMC article. Review.
-
Pattern recognition receptors: function, regulation and therapeutic potential.Signal Transduct Target Ther. 2025 Jul 11;10(1):216. doi: 10.1038/s41392-025-02264-1. Signal Transduct Target Ther. 2025. PMID: 40640149 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

