Bone metabolism and incretin hormones following glucose ingestion in young adults with pancreatic insufficient cystic fibrosis
- PMID: 36110921
- PMCID: PMC9467887
- DOI: 10.1016/j.jcte.2022.100304
Bone metabolism and incretin hormones following glucose ingestion in young adults with pancreatic insufficient cystic fibrosis
Abstract
Background: Gut-derived incretin hormones, including glucose-dependent insulinotropic peptide (GIP) and glucagon-like peptide 1 (GLP-1), regulate post-prandial glucose metabolism by promoting insulin production. GIP, GLP-1, and insulin contribute to the acute bone anti-resorptive effect of macronutrient ingestion by modifying bone turnover. Cystic fibrosis (CF) is associated with exocrine pancreatic insufficiency (PI), which perturbs the incretin response. Cross-talk between the gut and bone ("gut-bone axis") has not yet been studied in PI-CF. The objectives of this study were to assess changes in biomarkers of bone metabolism during oral glucose tolerance testing (OGTT) and to test associations between incretins and biomarkers of bone metabolism in individuals with PI-CF.
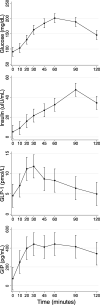
Methods: We performed a secondary analysis of previously acquired blood specimens from multi-sample OGTT from individuals with PI-CF ages 14-30 years (n = 23). Changes in insulin, incretins, and biomarkers of bone resorption (C-terminal telopeptide of type 1 collagen [CTX]) and formation (procollagen type I N-terminal propeptide [P1NP]) during OGTT were computed.
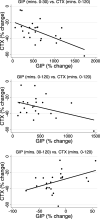
Results: CTX decreased by 32% by min 120 of OGTT (P < 0.001), but P1NP was unchanged. Increases in GIP from 0 to 30 mins (rho = -0.48, P = 0.03) and decreases in GIP from 30 to 120 mins (rho = 0.62, P = 0.002) correlated with decreases in CTX from mins 0-120. Changes in GLP-1 and insulin were not correlated with changes in CTX, and changes in incretins and insulin were not correlated with changes in P1NP.
Conclusions: Intact GIP response was correlated with the bone anti-resorptive effect of glucose ingestion, represented by a decrease in CTX. Since incretin hormones might contribute to development of diabetes and bone disease in CF, the "gut-bone axis" warrants further attention in CF during the years surrounding peak bone mass attainment.
Keywords: Bone; CF, cystic fibrosis; CTX, C-terminal telopeptide of type 1 collagen; Cystic Fibrosis; GIP, gastric inhibitory polypeptide; GLP-1, glucagon-like peptide-1; Incretins; Nutrition; OGTT; OGTT, oral glucose tolerance test; P1NP, procollagen type I N-terminal propeptide; PI, pancreatic insufficiency.
© 2022 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Effect of GIP and GLP-1 infusion on bone resorption in glucose intolerant, pancreatic insufficient cystic fibrosis.J Clin Transl Endocrinol. 2025 Apr 7;40:100392. doi: 10.1016/j.jcte.2025.100392. eCollection 2025 Jun. J Clin Transl Endocrinol. 2025. PMID: 40275940 Free PMC article.
-
Bone resorption and incretin hormones following glucose ingestion in healthy emerging adults.J Clin Transl Endocrinol. 2023 Feb 6;31:100314. doi: 10.1016/j.jcte.2023.100314. eCollection 2023 Mar. J Clin Transl Endocrinol. 2023. PMID: 36845829 Free PMC article.
-
Differential impact of glucose administered intravenously or orally on bone turnover markers in healthy male subjects.Bone. 2017 Apr;97:261-266. doi: 10.1016/j.bone.2017.01.027. Epub 2017 Jan 23. Bone. 2017. PMID: 28126633 Clinical Trial.
-
The evolving story of incretins (GIP and GLP-1) in metabolic and cardiovascular disease: A pathophysiological update.Diabetes Obes Metab. 2021 Sep;23 Suppl 3:5-29. doi: 10.1111/dom.14496. Diabetes Obes Metab. 2021. PMID: 34310013 Review.
-
The Gut-Bone Axis in Diabetes.Curr Osteoporos Rep. 2023 Feb;21(1):21-31. doi: 10.1007/s11914-022-00767-2. Epub 2022 Nov 28. Curr Osteoporos Rep. 2023. PMID: 36441432 Review.
Cited by
-
Gut hormones and bone homeostasis: potential therapeutic implications.Nat Rev Endocrinol. 2024 Sep;20(9):553-564. doi: 10.1038/s41574-024-01000-z. Epub 2024 Jun 10. Nat Rev Endocrinol. 2024. PMID: 38858581 Review.
-
Effect of GIP and GLP-1 infusion on bone resorption in glucose intolerant, pancreatic insufficient cystic fibrosis.J Clin Transl Endocrinol. 2025 Apr 7;40:100392. doi: 10.1016/j.jcte.2025.100392. eCollection 2025 Jun. J Clin Transl Endocrinol. 2025. PMID: 40275940 Free PMC article.
-
Bone resorption and incretin hormones following glucose ingestion in healthy emerging adults.J Clin Transl Endocrinol. 2023 Feb 6;31:100314. doi: 10.1016/j.jcte.2023.100314. eCollection 2023 Mar. J Clin Transl Endocrinol. 2023. PMID: 36845829 Free PMC article.
-
Cystic fibrosis-related bone disease: an update on screening, diagnosis, and treatment.Ther Adv Endocrinol Metab. 2025 Apr 2;16:20420188251328210. doi: 10.1177/20420188251328210. eCollection 2025. Ther Adv Endocrinol Metab. 2025. PMID: 40183033 Free PMC article. Review.
References
-
- Weaver C.M. “The role of nutrition on optimizing peak bone mass,” (in eng) Asia Pac J Clin Nutr. 2008;17(Suppl 1):135–137. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

