DNA damage response and repair genes in advanced bone and soft tissue sarcomas: An 8-gene signature as a candidate predictive biomarker of response to trabectedin and olaparib combination
- PMID: 36110934
- PMCID: PMC9469659
- DOI: 10.3389/fonc.2022.844250
DNA damage response and repair genes in advanced bone and soft tissue sarcomas: An 8-gene signature as a candidate predictive biomarker of response to trabectedin and olaparib combination
Abstract
Background: Advanced and unresectable bone and soft tissue sarcomas (BSTS) still represent an unmet medical need. We demonstrated that the alkylating agent trabectedin and the PARP1-inhibitor olaparib display antitumor activity in BSTS preclinical models. Moreover, in a phase Ib clinical trial (NCT02398058), feasibility, tolerability and encouraging results have been observed and the treatment combination is currently under study in a phase II trial (NCT03838744).
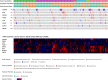
Methods: Differential expression of genes involved in DNA Damage Response and Repair was evaluated by Nanostring® technology, extracting RNA from pre-treatment tumor samples of 16 responder (≥6-month progression free survival) and 16 non-responder patients. Data validation was performed by quantitative real-time PCR, RNA in situ hybridization, and immunohistochemistry. The correlation between the identified candidate genes and both progression-free survival and overall survival was investigated in the publicly available dataset "Sarcoma (TCGA, The Cancer Genome Atlas)".
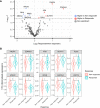
Results: Differential RNA expression analysis revealed an 8-gene signature (CDKN2A, PIK3R1, SLFN11, ATM, APEX2, BLM, XRCC2, MAD2L2) defining patients with better outcome upon trabectedin+olaparib treatment. In responder vs. non-responder patients, a significant differential expression of these genes was further confirmed by RNA in situ hybridization and by qRT-PCR and immunohistochemistry in selected experiments. Correlation between survival outcomes and genetic alterations in the identified genes was shown in the TCGA sarcoma dataset.
Conclusions: This work identified an 8-gene expression signature to improve prediction of response to trabectedin+olaparib combination in BSTS. The predictive role of these potential biomarkers warrants further investigation.
Keywords: DNA damage response and repair genes; bone and soft tissue sarcomas; olaparib; predictive biomarkers; trabectedin.
Copyright © 2022 Merlini, Centomo, Ferrero, Chiabotto, Miglio, Berrino, Giordano, Brusco, Pisacane, Maldi, Sarotto, Capozzi, Lano, Isella, Crisafulli, Aglietta, Dei Tos, Sbaraglia, Sangiolo, D’Ambrosio, Bardelli, Pignochino and Grignani.
Conflict of interest statement
GGr has received fees for consulting/advisory roles from PharmaMar, Lilly, Novartis, Bayer, and Eisai. LDA received travel grant from PharmaMar and Lilly. MA has received fees for consulting/advisory roles from Bristol- Myers Squibb, Merck, and Roche. AB served in a consulting/advisory role for Illumina and Inivata. AB is cofounder and shareholder of NeoPhore. AB is a member of the NeoPhore scientific advisory board. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Demetri GD, et al. Efficacy and safety of trabectedin or dacarbazine for metastatic liposarcoma or leiomyosarcoma after failure of conventional chemotherapy: Results of a phase III randomized multicenter clinical trial. J Clin Oncol (2016) 34(8):786–93. doi: 10.1200/JCO.2015.62.4734 - DOI - PMC - PubMed
-
- Judson I, et al. Randomised phase II trial of pegylated liposomal doxorubicin (DOXIL/CAELYX) versus doxorubicin in the treatment of advanced or metastatic soft tissue sarcoma: a study by the EORTC soft tissue and bone sarcoma group. Eur J Cancer (2001) 37(7):870–7. doi: 10.1016/S0959-8049(01)00050-8 - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

