Endocytosis of the thrombopoietin receptor Mpl regulates megakaryocyte and erythroid maturation in mice
- PMID: 36110936
- PMCID: PMC9468709
- DOI: 10.3389/fonc.2022.959806
Endocytosis of the thrombopoietin receptor Mpl regulates megakaryocyte and erythroid maturation in mice
Abstract
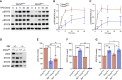
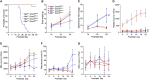
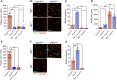
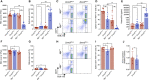
Dnm2fl/fl Pf4-Cre (Dnm2Plt-/- ) mice lacking the endocytic GTPase dynamin 2 (DNM2) in platelets and megakaryocytes (MKs) develop hallmarks of myelofibrosis. At the cellular level, the tyrosine kinase JAK2 is constitutively active but decreased in expression in Dnm2Plt-/- platelets. Additionally, Dnm2Plt-/- platelets cannot endocytose the thrombopoietin (TPO) receptor Mpl, leading to elevated circulating TPO levels. Here, we assessed whether the hyperproliferative phenotype of Dnm2Plt-/- mice was due to JAK2 constitutive activation or to elevated circulating TPO levels. In unstimulated Dnm2Plt-/- platelets, STAT3 and, to a lower extent, STAT5 were phosphorylated, but their phosphorylation was slowed and diminished upon TPO stimulation. We further crossed Dnm2Plt-/- mice in the Mpl-/- background to generate Mpl-/-Dnm2Plt-/- mice lacking Mpl ubiquitously and DNM2 in platelets and MKs. Mpl-/- Dnm2Plt-/- platelets had severely reduced JAK2 and STAT3 but normal STAT5 expression. Mpl-/- Dnm2Plt-/- mice had severely reduced bone marrow MK and hematopoietic stem and progenitor cell numbers. Additionally, Mpl-/- Dnm2Plt-/- mice had severe erythroblast (EB) maturation defects, decreased expression of hemoglobin and heme homeostasis genes and increased expression of ribosome biogenesis and protein translation genes in spleen EBs, and developed anemia with grossly elevated plasma erythropoietin (EPO) levels, leading to early fatality by postnatal day 25. Mpl-/- Dnm2Plt+/+ mice had impaired EB development at three weeks of age, which normalized with adulthood. Together, the data shows that DNM2-dependent Mpl-mediated endocytosis in platelets and MKs is required for steady-state hematopoiesis and provides novel insights into a developmentally controlled role for Mpl in normal erythropoiesis, regulating hemoglobin and heme production.
Keywords: dnm2; erythropoiesis; hematopoiesis; megakaryopoeiesis; mpl.
Copyright © 2022 Eaton, Boyd, Biswas, Lee-Sundlov, Dlugi, Ramsey, Zheng, Burns, Sola-Visner, Hoffmeister and Falet.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Dynamin 2-dependent endocytosis is required for normal megakaryocyte development in mice.Blood. 2015 Feb 5;125(6):1014-24. doi: 10.1182/blood-2014-07-587857. Epub 2014 Dec 2. Blood. 2015. PMID: 25468568 Free PMC article.
-
Thrombopoietin induces phosphoinositol 3-kinase activation through SHP2, Gab, and insulin receptor substrate proteins in BAF3 cells and primary murine megakaryocytes.J Biol Chem. 2001 Jan 26;276(4):2494-502. doi: 10.1074/jbc.M002633200. Epub 2000 Oct 27. J Biol Chem. 2001. PMID: 11054408
-
Dynamin 2 is required for GPVI signaling and platelet hemostatic function in mice.Haematologica. 2020 May;105(5):1414-1423. doi: 10.3324/haematol.2019.218644. Epub 2019 Jul 11. Haematologica. 2020. PMID: 31296575 Free PMC article.
-
Thrombopoietin and the c-Mpl receptor: insights from gene targeting.Int J Biochem Cell Biol. 1999 Oct;31(10):1027-35. doi: 10.1016/s1357-2725(99)00079-5. Int J Biochem Cell Biol. 1999. PMID: 10582337 Review.
-
Studies of the c-Mpl thrombopoietin receptor through gene disruption and activation.Stem Cells. 1996;14 Suppl 1:124-32. doi: 10.1002/stem.5530140716. Stem Cells. 1996. PMID: 11012212 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

